Impact of Next Generation Imaging: What Is the Optimal (Current) Tracer for PET-Based Imaging for Staging Presentation - Ken Herrmann
August 2, 2022
Biographies:
Ken Herrmann, MD, MBA, Professor and Chair of the Department of Nuclear Medicine, Universitatsklinikum Essen, Essen, Germany
The Mechanism of PSMA PET for Prostate Cancer - Stefano Fanti and Ken Herrmann
APCCC 2021: PSMA-Based Imaging: Navigating the Pitfalls of the Different Tracers
(UroToday.com) The 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Hybrid Meeting included a session on high-risk and locally advanced prostate cancer and a presentation by Dr. Ken Herrmann discussing the impact of next generation imaging and the optimal tracer for PET-based imaging for staging. To set the stage, Dr. Herrmann notes that we are nearly a decade since the first report of human application of PSMA PET/CT, with several available 68Ga-labeled PSMA ligands (68Ga-PSMA-11, 68Ga-PSMA-I&T) and 18F-labeled PSMA ligands (18F-DCFBC, 18F-DCFPyL, 18F-PSMA-1007, 18F-rhPSMA7):
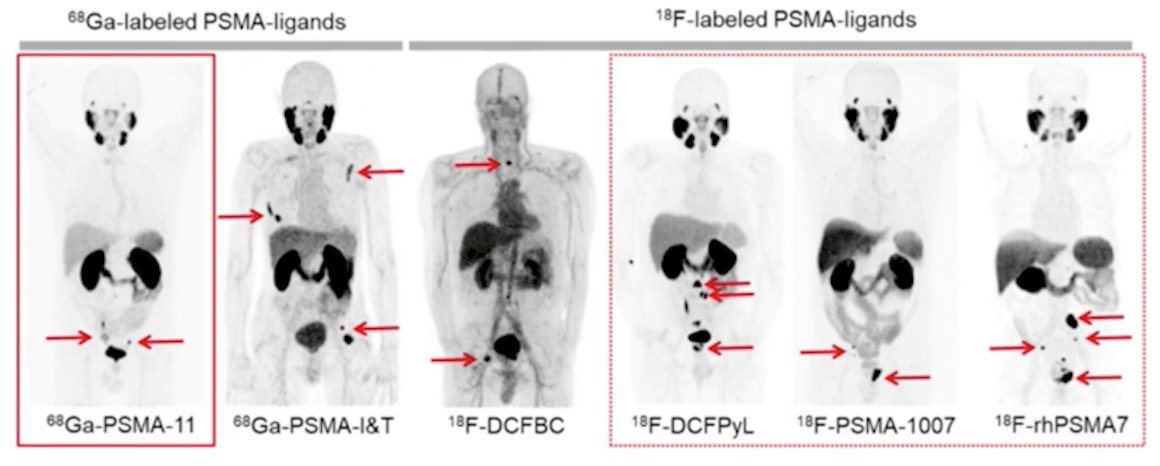
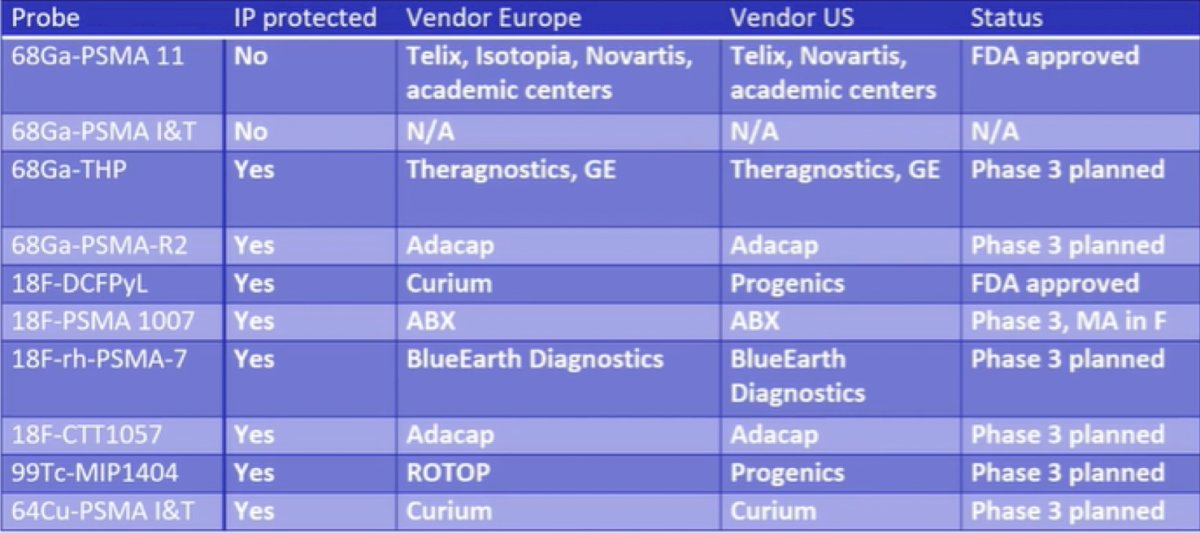
Dr. Herrmann notes that the majority of his discussion focuses on 68Ga-PSMA-11, 18F-DCFPyL and 18F-PSMA-1007, with the overall current PSMA PET/CT landscape as follows:
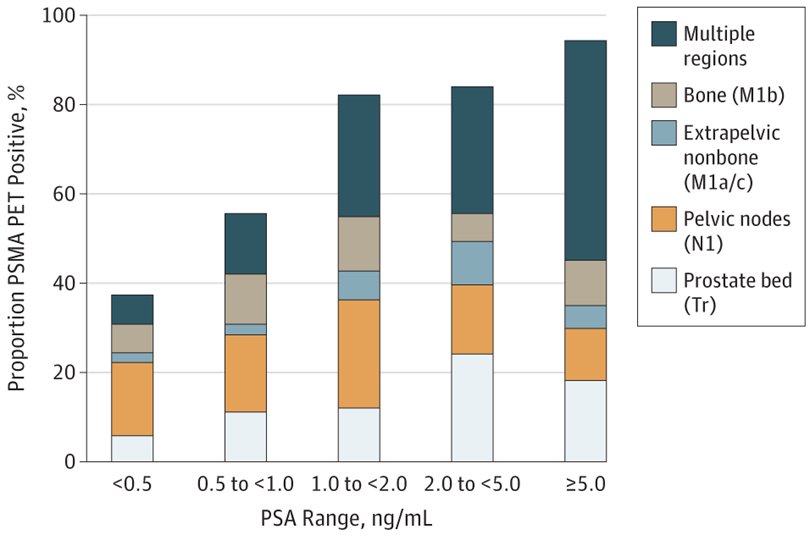
Dr. Herrmann emphasied that there is excellent evidence for both 68Ga-PSMA and 18F-PSMA PET/CT. Hope et al.1 published in 2021 a phase 3 imaging trial assessing the accuracy of 68Ga-PSMA-11 PET imaging for the detection of pelvic nodal metastases compared with histopathology at time of radical prostatectomy and pelvic lymph node dissection. In this trial, there were 764 men that underwent a 68Ga-PSMA-11 PET imaging scan for primary staging, and 277 of 764 (36%) subsequently underwent prostatectomy with lymph node dissection. Based on pathology reports, 75 of 277 patients (27%) had pelvic nodal metastasis. Results of 68Ga-PSMA-11 PET/CT were positive in 40 of 277 (14%), 2 of 277 (1%), and 7 of 277 (3%) of patients for pelvic nodal, extrapelvic nodal, and bone metastatic disease, respectively. Sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases were 0.40 (95% CI, 0.34-0.46), 0.95 (95% CI, 0.92-0.97), 0.75 (95% CI, 0.70-0.80), and 0.81 (95% CI, 0.76-0.85), respectively. Fendler and colleagues2 also assessed 68Ga-PSMA-11 PET/CT accuracy in a prospective multicenter trial among 635 patients with biochemically recurrent prostate cancer after prostatectomy (n = 262, 41%), radiation therapy (n = 169, 27%), or both (n = 204, 32%). On a per-patient basis, positive predictive value was 0.84 (95% CI, 0.75-0.90) by histopathologic validation and 0.92 (95% CI, 0.88-0.95) by the composite reference standard (n = 217), with 68Ga-PSMA-11 PET/CT localizing recurrent prostate cancer in 475 of 635 (75%) patients. The detection rates stratified by PSA are as follows:
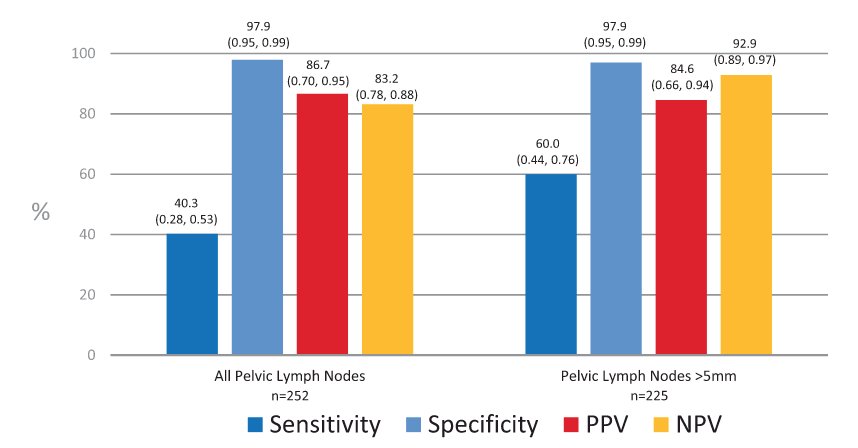
With regards to 18F-DCFPyL PSMA PET/CT, there is evidence from both the CONDOR3 and OSPREY4 clinical trials. In CONDOR, patients were recruited that had biochemically recurrent disease and rising PSA after definitive therapy and negative or equivocal standard of care imaging (e.g., CT/MRI, bone scintigraphy, or F-18 fluciclovine). Patients with positive 18F-DCFPyL-PET/CT scans based on local interpretation were scheduled for follow up within 60 days to verify suspected lesion(s) using a composite standard of truth. As their primary outcome of interest, the authors assessed the correct localization rate (CLR), defined as percentage of patients with a 1:1 correspondence between at least one lesion identified by DCFPyL-PET/CT and the composite standard of truth: pathology, correlative imaging, or PSA response, in descending order of priority. Among 208 men, the median PSA was 0.8 [0.2 - 98.4] ng/mL, and the primary outcome of correct localization rate for DCFPyL-PET/CT was 84.8-87.0% of cases among the three readers (lower bound of 95% CI: 77.8%-80.4%), against the composite standard of truth. The OSPREY trial was a prospective trial designed to determine the diagnostic performance of 18F-DCFPyL-PET/CT for detecting sites of metastatic prostate cancer. Cohort A enrolled men with high-risk prostate cancer undergoing radical prostatectomy with pelvic lymphadenectomy. In this cohort of 252 patients, 18F-DCFPyL-PET/CT had median specificity of 97.9% (95% CI: 94.5%-99.4%) and median sensitivity of 40.3% (28.1%-52.5%, not meeting prespecified end point) among 3 readers for pelvic nodal involvement. Additionally, the median positive predictive value and negative predictive value were 86.7% (69.7%-95.3%) and 83.2% (78.2%-88.1%), respectively:
Additional evidence for PSMA PET/CT is provided by the proPSMA study.5 In this trial, 302 men were randomly assigned to conventional imaging (n=152) and PSMA PET-CT (n=150). PSMA PET-CT had a 27% (95% CI 23-31) greater accuracy than that of conventional imaging (92% [88-95] vs 65% [60-69]; p<0.0001). Additionally, this trial found a lower sensitivity (38% [24-52] vs 85% [74-96]) and specificity (91% [85-97] vs 98% [95-100]) for conventional imaging compared with PSMA PET-CT.
Dr. Herrmann notes that there are pros and cons to 68Ga-PSMA-11, 18F-DCFPyL and 18F-PSMA-1007. For 68Ga-PSMA-11, the pros are (i) that proPSMA was done with 68Ga-PSMA-11, (ii) the overall majority of data is from this tracer, and (iii) it is widely available (FDA approved, 2 EMA submissions pending). The two main cons of 68Ga-PSMA-11 are that there are logistic challenges and that it is excreted via the kidneys and urinary tract. For 18F-DCFPyL, the pros are (i) it is FDA approved, (ii) the OSPRETY trial provides evidence in primary staging, and (iii) there are logistical advantages. The two main cons of 18F-DCFPyL are that it is excreted via the kidneys and urinary tract and there is less data than for 68Ga-PSMA-11. For 18F-PSMA-1007, the pros are that it is predominantly excreted via the GI tract and there is market approval in France, whereas the cons are that it has the least data among the three front runners and unspecific bone uptake is a major challenge (~50%).
Dr. Herrmann concluded his presentation by discussing the impact of next-generation imaging and the optimal tracer for PET-based imaging for staging with the following take home messages:
- PSMA PET/CT is implemented into the clinical guidelines (NCCN, EAU)
- Differences between PSMA PET/CT tracers exist
- Most data so far are available for PSMA-11 and PyL, with both mainly being excreted via the kidneys
- PSMA-1007 is predominantly excreted via the GI tract, but there are challenges of unspecific bone uptake
Presented By: Ken Herrmann, MD, MBA, University of Duisburg-Essen, University Hospital Essen, Essen, Germany
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Advanced Prostate Cancer Consensus Conference (APCCC) Annual Hybrid Meeting, Lugano, Switzerland, Thurs, Apr 28 – Sat, Apr 30, 2022.
References:
- Hope TA, Eiber M, Armstrong WR, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021 Nov 1;7(11):1635-1642.
- Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol 2019 Jun 1;5(6):856-863.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Feb 23 [Epub ahead of print].
- Pienta KJ, Gorin MA, Rowe SP, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021 Jul;206(1):52-61.
- Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020 Apr 11;395(10231):1208-1216.


