SER120 was approved by the FDA in March 2017 for the treatment of nocturia due to nocturnal polyuria in adults with 2 or more nocturic voids per night. The tradename for SER120 (AV002) is NoctivaTM. Nocturia is a highly prevalent voiding disorder with numerous underlying contributing factors. Although the ICS definition for nocturia is one or more nighttime voids interrupting sleep, for purposes of drug development and clinical treatment, the FDA uses the definition of 2 or more such voids which is the inflection point for causing impactful disturbances of sleep, impaired quality of life, increased risk of falls and fractures and other adverse medical consequences. In published studies of nocturia in Japan, North America and Europe1, 2, 3, the correlation of nocturia (nighttime frequency) and nocturnal polyuria (excess nighttime volume) is approximately 80%. The clinical studies of SER120 showed the same substantial overlap of nocturia patients due to nocturnal polyuria.
SER120 (NoctivaTM) is the first and only drug approved by the FDA specifically for treatment of nocturia. Noctiva is a vasopressin analog which binds to the V2 receptors of the renal collecting ducts and stimulates reabsorption of water from the dilute urine in the lumen to the systemic circulation. This concentrates the urine, reduces volume and decreases the frequency of nocturic voids. Since 80% or more of patients with nocturia also have nocturnal polyuria the pharmacology of Noctiva is well-suited to target the most important underlying factor contributing to the nocturia in the vast majority of patients. Although some patients with nocturia will have overactive bladder or benign prostatic hyperplasia as contributing factors, 80% of these patients also have nocturnal polyuria. The drugs used to treat OAB and BPH do not address the nocturnal polyuria and, therefore, have been relatively ineffective for nocturia even in patients with these conditions.4,5 This is because antimuscarinics, beta-3 agonists, alpha blockers and 5 alpha reductase inhibitors target the detrusor muscle of the urinary bladder and the prostate, respectively, rather than the kidneys.
Behavioral modification for nocturia which involves permanent fluid restriction, avoidance of alcohol and caffeine and changes in the timing of eating of drinking have shown some beneficial effects in short-term studies but have not proven to have durable efficacy and are not sustainable in real life for most patients.6 Other therapeutic approaches for nocturia have included changing the times in which diuretics are administered or attempting to use diuretics to stimulate voiding prior to sleep. The evidence to support this approach is quite limited and the pharmacokinetic and pharmacodynamic variability of these drugs makes this an unreliable therapy.
The problem with using a vasopressin analog for nocturia in the past has been the risk of hyponatremia due to an ongoing anti-diuretic effect extending into the day when the patient resumes drinking fluids. This can result in water-loading with the expansion of the intravascular volume and consequent dilution of serum electrolytes including sodium. The challenge has been to confine the anti-diuretic effect just to the night. NoctivaTM overcomes this problem because it is a novel, engineered dosage form of desmopressin designed to have a precise and consistent pharmacokinetic profile which limits its duration of effect to approximately 4 to 6 hours while the patient is sleeping. It contains very low doses of desmopressin, 0.83 mcg and 1.66 mcg which are approximately 10 times lower than the existing nasal spray product and 100 times lower than the desmopressin oral tablet. The much lower dose is necessary but not sufficient to meet this challenge. NoctivaTM is the only emulsified formulation of desmopressin. It contains a patented permeation enhancer, CPD (cyclopentadecanolide) which widens the gaps between nasal mucosal cells enabling much more rapid, complete and consistent absorption of the desmopressin peptide into the blood. In Phase 1 pharmacokinetic study at the 2 mcg dose, the mean Tmax of Noctiva is approximately 20 minutes which is more rapid than a bolus subcutaneous injection of desmopressin. There is almost no depot effect. The PK coefficient of variation is in the range of a subcutaneous or intradermal injection. This consistent PK profile produces a predictable and reliable peak plasma concentration below 10 pg/mL which, in turn, limits the duration of the anti-diuretic effect to the nighttime hours and substantially decreases the risk of hyponatremia.
The NoctivaTM clinical development program consisted of 10 studies including 4 Phase 3 trials and 2 long-term extension studies with up to 2 years of continuous treatment. Over 2300 patients were enrolled and evaluated. The pivotal studies and safety extension trials attempted to evaluate a representative cross-section of the actual target patient population with nocturia in close to real-world conditions. There were no restrictions on fluids, alcohol or caffeine. Patients were instructed to continue their lifestyle habits prior to the study. The eligibility criteria accepted patients with numerous concomitant medical conditions typical of an older patient population including hypertension, treatment with thiazide diuretics, diabetes mellitus, mild congestive heart failure (NYHA Class I), OAB (with or without treatment) and BPH (with or without treatment). Concomitant high dose systemic corticosteroids were not allowed because of the increased risk of water retention and hyponatremia but nasal sprays containing steroids were permitted. At the FDA’s request, only patients age 50 years and older were enrolled to enrich the safety population with older patients who were at greater risk for hyponatremia. Approximately 55% of patients were 65 years or older and approximately 21% were 75 years or older. Patients with and without nocturnal polyuria were enrolled in these trials.
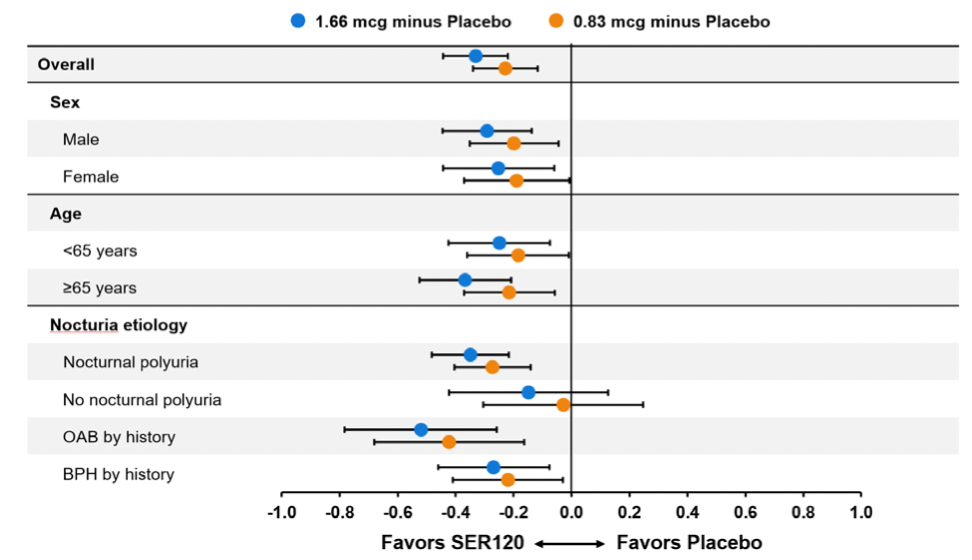
In addition to the results reported in the Journal of Urology abstract, other analyses were conducted which provide important information concerning the use of NoctivaTM for nocturia. The pivotal studies were sufficiently large to allow subpopulation analyses. Figure 1 below is a forest plot of the reduction in the mean number of nocturic voids per night for each dose group of Noctiva relative to placebo for patient subgroups including males and females, patients less than 65 years of age and ≥ 65 years old and various contributing nocturia etiologies. The results are statistically significant for both doses of NoctivaTM for each subgroup with the exception of patients with no nocturnal polyuria. However, this group of patients still showed to have numerical trends which favor Noctiva.
Figure 1: Co-primary Efficacy Variable 1 – Reduction in Mean Nocturic Episodes by Gender, Age and Nocturia Etiology ISE DB3/DB4 Studies, ITT Population
An additional secondary efficacy endpoint was the time from going to bed to the first nocturic void. This is the first period of uninterrupted sleep. Published papers have shown that the first 4 hours of uninterrupted sleep is correlated with better functioning in terms of the activities of daily living.7 Both doses of NoctivaTM were statistically significant relative to placebo and produced the approximately 4 hours of sleep considered to be a threshold for better functioning. (Figure 2)
Figure 2: Secondary Efficacy Variable 1 – Change in Time from Bedtime to First Nocturic Episode ISE DB3/DB4 Studies, ITT Population

The definition of responder which was used in the NoctivaTM program was rigorous and required a ≥ 50% reduction in the mean number of nocturic voids from baseline. Other programs used a ≥33% reduction. Approximately 50% of patients receiving the 1.66 mcg dose of NoctivaTM met the responder criterion and over 70% met the ≥ 33% criterion.
Figure 3 below depicts the improvements in various primary and secondary efficacy endpoints which the average responding patient experienced. Of note, there was a reduction of 2.1 nocturic voids per night which is 15 fewer episodes per week. Seventy-six percent of nights had ≤ 1 nocturic void. The initial period of uninterrupted sleep was over 5 hours and the reduction in nocturnal urine volume was 330 mL.
Figure 3: Magnitude of Change in Responders for Various Efficacy Variables in 1.66 mcg Treatment Group (N=214)

The onset of action of NoctivaTM occurs with the first dose as shown in Figure 4 below.
Figure 4: SER120 Immediate Pharmacological Effects on Reduction of Nocturic Voids
Each dose is independent and no steady state blood concentration is required for efficacy. In fact, NoctivaTM is designed not to accumulate in the blood and each dose is eliminated before the next dose is given. The efficacy of NoctivaTM can be assessed in the first week of treatment. The long- term extension studies documented that there is no tachyphylaxis and that the anti-diuretic pharmacology and therapeutic efficacy of NoctivaTM is durable over 2 years of continuous treatment as shown in Figure 5 below.
Figure 5: Long-term Efficacy Assessment of SER120 on the Reduction of Nocturic Void in Two Open-Label Trials

Written by: Seymour Fein, MD, Chief Medical Officer, a principal and co-founder of Serenity Pharmaceuticals, board-certified in both oncology and internal medicine.
Read the Abstract
References:
1. Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322-330.
2. Chang SC, Lin AT, Chen KK, Chang LS. Multifactorial nature of male nocturia. Urology 2006;67(3):541-544.
3. Weiss JP. Prevalence of Nocturnal polyuria in nocturia. J. Urol. 2009;181(4):538
4. Sakalis VI, Karavitakis M, Bedretdinova D, et al. Medical treatment of nocturia in men with lower urinary tract symptoms: a systematic review by the European Association of Urology Guidelines Panel for Male Lower Urinary Tract Symptoms. Eur Urol 2017:72(5):757-769.
5. Van Kerrebroeck P, Drake M, Rees J. Nocturia: what do we need to know in 2017? Identify the cause and tailoring the treatment. Eur Med J Urol. 2017;5(1):32-37.
6. Weiss JP, Blaivas JG, Bliwise DL, Dmochowski RR, Dubeau CE, Lowe FC, Petrou SP. Van Kerrebroeck P, Rosen RC, Wein, AK. The evaluation and treatment of nocturia; a consensus statement. BJU Int. 2011 Jul;108(1):6-21
7. Stanley, N. The underestimated impact of nocturia on quality of life. Eur Urol Suppl 2005;4(7):17-19


