Presented by the Warren Alpert Medical School of Brown University, Office of Continuing Medical Education.
Target Audience
This activity is designed for urologists and other healthcare professionals interested in or involved with the management of bladder cancer.
Educational Objectives
Upon completion of this activity, participants should be able to:
- Discuss the epidemiology of bladder cancer including prevalence, mortality, and risk factors
- Describe the economic impact of bladder cancer and how improved techniques for initial detection and at the time of transurethral resection of bladder tumor (TURBT) may decrease the economic burden
- Compare and contrast new urine biomarkers when compared to urine cytology
- Discuss the role of photodynamic diagnosis using hexaminolevulinate-guided fluorescence cystoscopy in detecting carcinoma in situ and residual disease at the time of TURBT compared to traditional white-light cystoscopy (WLC)
- Optimize use of intravesical therapy after TURBT to prevent recurrence of bladder cancer and treat patients who do not respond to bacillus Calmette-Guérin (BCG) therapy
- Discuss potential future treatment methods for patients with bladder cancer
Faculty
Pamela I. Ellsworth, MD, FACS, Program Chair
Associate Professor of Surgery
Division of Urology
The Warren Alpert Medical School of Brown University
Providence, Rhode Island
Muhammad Choudhury, MD, FACS
Professor and Chairman
Department of Urology
New York Medical College
Valhalla, New York
Support Acknowledgement
This activity is supported by educational grants from ENDO Pharmaceuticals and GE Healthcare.
Faculty Disclosures
In accordance with the disclosure policy of the Warren Alpert Medical School of Brown University as well as standards set forth by the Accreditation Council for Continuing Medical Education, all speakers and individuals in a position to control the content of a CME activity are required to disclose relevant financial relationships with commercial interests (within the past 12 months). Disclosures of this activity’s speakers and planning committee have been reviewed and all identified conflicts of interest, if applicable, have been resolved.
Pamela I. Ellsworth, MD, is a consultant for Allergan, Inc. and Pfizer Inc; she is a speaker for Pfizer Inc; she has participated in speaker training for Novartis Pharmaceuticals Corporation; and she has participated in clinical research studies sponsored by Novartis Pharmaceuticals Corporation and Pfizer Inc.
Muhammad Choudhury, MD, has disclosed no relevant financial relationships. He does intend to discuss off-label uses of drugs, mechanical devices, biologics, or diagnostics approved by the FDA for use in the United States in his presentation.
Accreditation Statement
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the Warren Alpert Medical School of Brown University and Health and Wellness Education Partners. The Alpert Medical School is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation Statement
The Alpert Medical School designates this enduring material for a maximum of 1.5 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
AAPA Credit
AAPA accepts certificates of participation for educational activities certified for Category 1 credit from AOACCME, prescribed credit from AAFP, and AMA PRA Category 1.5 Credits™ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.5 hour of Category 1 credit for completing this program.
Program Release: September 1, 2011
Program Expiration: September 30, 2012
Estimated time to complete: 90 minutes
There are no prerequisites for participation.
Method of Participation and How To Receive CME Credit
- There are no fees for participating in and receiving credit for this activity.
- Review the activity objectives, faculty information, and CME information prior to participating in the activity.
- Complete the CME Activity.
- For receipt of CME credit, complete the CME posttest and activity evaluation at the conclusion of the activity.
- A minimum score of 80% is required to receive a CME credit certificate.
- Your CME credit certificate will be emailed to you within four weeks of activity completion.
- To complete the posttest and evaluation for this activity and receive CME credit, click here.
Privacy Policy
The Office of Continuing Medical Education (CME) and its educational partners protect the privacy of personal and other information regarding participants and educational collaborators. The CME Office maintains its Internet site as an information resource and service for physicians, other health professionals, and the public. The CME Office will keep your personal information confidential when you participate in a CME Internet-based program. CME collects only the information necessary to provide you with the services that you request.
Disclaimer
The opinions and recommendations expressed by faculty and other experts whose input is included in this activity are their own. This enduring material is produced for educational purposes only. The use of the Warren Alpert Medical School of Brown University name implies review of educational format, design, and approach. Activity participants have the professional responsibility to review the complete prescribing information of specific drugs or combination of drugs including indications, contraindications, warnings, and adverse effects before administering pharmacologic therapy to patients. The Warren Alpert Medical School assumes no liability for the information herein.
To Receive CME Credit
To complete the posttest and evaluation for this activity and receive CME credit, click here.
Introduction
As the fourth most common malignancy in the United States, bladder cancer results in nearly 15,000 deaths annually. Bladder cancer has the highest lifetime treatment costs per patient of all cancers, followed by colorectal, breast, prostate, and lung cancers. The tremendous human, psychological, and economic burden that follow a bladder cancer diagnosis underscores the importance of optimizing diagnostic and treatment protocols.
In this expert interview, UroToday® faculty experts—Pamela I. Ellsworth, MD, FACS, and Muhammad Choudhury, MD, FACS—will address some questions and challenges regarding the detection and initial treatment of bladder cancer.
Q1
UroToday: How common is bladder cancer? What are the trends in incidence and mortality of bladder cancer?
Bladder cancer is a common malignancy affecting both men and women, but men more commonly. In US men, bladder cancer is the fourth most common cancer and ranks eighth among cancer-related causes of death (Figure 1) [1]. It has been estimated that in 2011 there will be 52,020 new cases of bladder cancer in men and 17,230 new cases in women. In addition, nearly 15,000 deaths related to bladder cancer will occur in men and women in 2011 [2]. Based on rates from 2005 to 2007, the lifetime risk for being diagnosed with bladder cancer among men and women born today is 2.39%; 1.17% of men will develop bladder cancer between 50 and 70 years of age compared to 0.34% of women [3].
Figure 1. Leading Sites of New Cancer Cases and Deaths—2011 Estimates [1] Enlarge Image

American Cancer Society. Cancer Facts and Figures 2011. Atlanta: American Cancer Society, Inc.
Surveillance Epidemiology and End Results (SEER) incidence data from 2004 to 2008 demonstrated that the median age at diagnosis for bladder cancer was 73 years, and white men and women had a higher incidence compared with black, Asian/Pacific Islander, American Indian/Alaska Native, and Hispanic men and women [2]. Similarly, death rates by race were higher among white men; however, black women had a higher death rate than white women (2.7/100,000 vs 2.2/100,000). Bladder cancer incidence declined in women from 2004 to 2008, although there has been no change in the bladder cancer rate among men from 1986 to 2008. There appears to be a decrease in the mortality trend for both men and women from 1977 to 2007 [2].
Q2
UroToday: Bladder cancer management is known to be highly resource intensive. Will new techniques in bladder cancer detection and initial treatment impact the serious economic impact of bladder cancer?
Bladder cancer is associated with the highest lifetime treatment cost per patient of all cancers, followed by colorectal, breast, and lung cancer [3,4]. Bladder cancer also has the fifth highest overall cost, estimated at 3.4 billion dollars annually with 2.9 billion related to direct treatment-related costs per patient [4,5]. Avritscher and colleagues estimated that the lifetime mean treatment cost was $99,270 for muscle-invasive bladder cancer (MIBC) patients and $120,684 for non-muscle-invasive bladder cancer (NMIBC) patients [6]. The annual average of hospitalization days per year for patients with MIBC is more than 27 times higher than for patients with NMIBC (36 vs 1.3 days, respectively) [6]. In this study, the mean cost of bladder cancer was $65,158; 50% of the total cost ($32,559) was attributable to admissions and surgical procedures, whereas surveillance for and treatment of local recurrences accounted for 60% ($39,393). Also, 30% of the total cost ($19,811) was related to treatment of complications, 26% ($16,934) for MIBC, and 4% ($2312) for NMIBC [4,6].
The economic impact of disease progression is also significant. Treatment costs for patients with MIBC are nearly 3 times those for patients with stage Tis/Ta, and twice those for patients with T1 bladder cancer [4].
Several strategies have been proposed for reducing the economic burden of bladder cancer:
- Use of urine-based markers to identify incident or recurrent tumors earlier [7];
- Use of outpatient facilities for transurethral resection of bladder tumor (TURBT) reducing hospitalizations if surgical risks can be minimized [7]; and
- Improvement in the efficacy of intravesical treatments.
In addition, photodynamic diagnosis (PDD) has been proposed as a tool to improve the efficacy of initial TURBT and potentially decrease the residual risk for NMIBC [4,8,9]. TURBT is the largest bladder cancer expenditure and accounts for 71% of treatment costs in the United Kindgom [7]. The quality and result of the initial TURBT can have a significant effect on the bladder cancer patient’s overall prognosis and treatment costs. Several studies have concluded that the use of PDD can help to reduce the cost of bladder cancer treatment.
Daniltchenko and colleagues hypothesized that the initial PDD costs can be offset by a reduction in the number of TURBT follow-up visits [10]. In a prospective series of 115 patients without adjuvant treatment, recurrence rates after 5 years were 50% after PDD and 75% after white-light cystoscopy (WLC). It was estimated that, over a 5-year period, use of PDD avoided at least 20 additional TURBTs. The authors concluded that the benefits of PDD outweighed the initial costs [10]. Similarly, 2 European studies have demonstrated an economic benefit with use of PDD. Malmstrom and colleagues concluded that use of PDD in high-risk patients could save as much as 500,000 euros ($665,000) in the first year alone. Further, if PDD were used for all TURBTs in high- and medium-risk patients, the first year savings would be approximately 450,000 euros ($536,650) and 363,000 euros ($482,790), respectively [11]. A German study also demonstrated that PDD significantly reduces costs related to recurrent NMIBC [12].
Early detection of incident and recurrent disease has a key role in decreasing the risk and cost of disease progression [4]. Currently, WLC is the primary approach for diagnosis and surveillance of bladder cancer. It is estimated, however, that 10% to 20% of bladder tumors are missed during WLC [13]. Urine cytology might help with detection of bladder cancer; however, it lacks the immediate result warranted during TUR. Emerging technologies such as PDD and adjuvant intravesical therapies including mitomycin C and bacillus Calmette-Guérin (BCG) may help to improve the diagnosis of bladder cancer and decrease the risk for recurrence and progression [4].
Q3
UroToday: Although cystoscopy is the “gold standard” for the detection of bladder cancer, it is costly and invasive. What are the advantages and disadvantages of various urine markers for diagnosing or monitoring bladder cancer?
Flexible cystoscopy remains the primary diagnostic tool for detecting bladder cancer because it confers a low risk for infection and injury, causes minimal discomfort, and can be performed routinely in the physician’s office under local anesthesia. However, the procedure provokes anxiety in many patients, visibility can be reduced by bleeding, and small tumor and flat lesion (eg, carcinoma in situ) can be difficult to distinguish from normal tissue [14,15]. For these reasons, use of adjunctive urine tumor markers as in urine cytology can assist in identifying occult cancer. Cytology is used commonly to monitor tumor recurrence in patients with a previous diagnosis of bladder cancer, in the initial assessment of patients who present with hematuria or irritative voiding symptoms to rule out (or in) bladder tumor, and following TURBT to assess the adequacy of treatment [16]. Unfortunately, the usefulness of urine cytology is limited by its low sensitivity especially in low-grade NMIBC [17].
In recent years, numerous urine-based biomarkers have been evaluated to determine whether these tests can complement or replace the existing “gold standard” cystoscopy and urine cytology in monitoring patients at risk for recurrent bladder cancer.
An ideal biomarker for urothelial cancer should have high sensitivity and specificity and should be based on a voided urine specimen, cost effective, easy to interpret, and available as a point-of-care test.
URINE BIOMARKERS
The biomarkers shown in Table 1 are FDA approved and available for clinical use. A brief review of the molecular basis for the test, published results, and the advantages and disadvantages of each test is provided.
|
Table 1. Current FDA-Approved and Commercially Available Bladder Tumor Markers
FISH, fluorescent in situ hybridization. |
Nuclear Matrix Protein 22 (NMP22)
Nuclear matrix proteins (NMPs)are part of the mitotic apparatus of the cell, which consists of a 3-dimensional web of RNA and protein that supports the nuclear shape; organizes DNA; and coordinates DNA replication, transportation, and gene expression [18,19]. NMP22 may be present at up to 25-fold higher concentration in tumor cells than in normal urothelial cells [20,21]. NMP22 is released from the nuclei of the tumor cell during apoptosis into the urine and the level can be measured at point of care.
Advantages
In a 15-study meta-analysis, the reported sensitivity of NMP22 was 73%, compared with 34% for urine cytology [22]. Other studies have reported estimated sensitivity of NMP22 ranging from 32% to 100% when the test was used alone or in conjunction with urine cytology for surveillance of bladder tumor [23]. Overall, NMP22 has a higher sensitivity than urine cytology especially in the detection of low-grade and low-stage bladder tumors.
The point-of-care NMP22® BladderChek® test has shown sensitivities ranging from 40% to 90% [24,25,26,27,28]. In addition, the point-of-care test does not require expert analysis or laboratory time, is not dependent on intact cells, and provides unambiguous results [24].
Disadvantages
The same studies point out the lower specificity of NMP22 when compared with urine cytology. The median specificity in a meta-analysis was 80% compared to 99% for cytology [22]. Major sources of false positive results with NMP22 are related to hematuria and pyuria common with many benign urologic conditions [29].
Multitarget Multicolor FISH Assay
Chromosomal abnormalities in urothelial tumors can be detected by fluorescent in situ hybridization (FISH) using DNA probes to chromosome centromeres or unique loci that are altered in tumor cells [30].
The Vysis® UroVysion test is a multi-target, multicolored FISH assay that uses probes to identify aneuploidy of chromosomes 3, 7, and 17, combined with a probe to the 9p21 locus. This combination of probes has been shown to have the best sensitivity and specificity [31].
Advantages
Most studies report high specificity with the Vysis UroVysion test, ranging between 75% and 100%, which is comparable to cytology [30,32,33,34,35,36,37]. The test appears to maintain its high specificity among patients with a variety of benign urology conditions including microhematuria, benign prostatic hyperplasia (BPH), infection, and inflammation [34,38,39].
The test may also be able to predict tumor recurrence before urinary cytology. Skacel and colleagues reported that 8 of 9 patients with positive FISH test, atypical cytology, and negative bladder biopsy had biopsy-proven recurrence of bladder cancer within 12 months [40].
Overall, the specificity of urine FISH test is high and comparable to that of cytology. FISH testing may also predict tumor recurrence before cytology becomes abnormal. Thus, it may prove useful in surveillance for recurrent tumor in patients with normal cytology. The test can also be used as a confirmatory test for patients with atypical cytology.
Disadvantages
Most recent studies have reported sensitivity of the Vysis UroVysion test in the range of 30% to 86% [32,41,42,43]. The assay has increased sensitivity for detecting higher grade and higher stage tumors, however, the sensitivity for detecting lower grade tumors is not clear [30]. Lack of consensus on the criteria used to evaluate abnormal urothelial cells, relatively high cost and the need for specialized laboratory to perform the test are other drawbacks.
ImmunoCyt
The ImmunoCyt™ test is a combination of cytology and immunofluorescent assay. Using monoclonal antibodies, the test detects tumor-associated antigens that are present in urothelial carcinoma. Two fluorescent labeled monoclonal antibodies are targeted at mucin antigens expressed on most bladder cancer cells but not on normal transitional epithelial cells. A third probe detects a glycosylated form of high molecular carcinoembryonic antigen that is present in bladder cancer cells [44,45].
Advantages
The ImmunoCyt test for bladder tumor has a reported sensitivity of 67% to 100% for all tumors, which is an improvement over conventional cytology [30,44,45,46,47,48,49,50]. One of the 3 antibodies used in this test appears to be quite sensitive for low-grade tumor cells and thus may offer an important advantage for detecting low-grade tumors [30].
Disadvantages
The main drawback of the ImmunoCyt test is its low specificity—63% compared with 97% for cytology and 90% for Vysis UroVysion [30]. Moreover, the interpretation of the test may be difficult for many cytologists, and false-positive results have been reported in patients with urinary tract obstruction secondary to stone or BPH.
Bladder Tumor Antigen (BTA)
The qualitative point-of-care test BTA stat® and the quantitative BTA-TRAK assays detect human complement factor H–related protein. BTA stat is an immunoassay that can be performed in 5 minutes, whereas BTA TRAK® is a standard enzyme-linked immunosorbent assay (ELISA) that quantitatively measures the amount of complement factor H–related protein and complement factor H in urine [51].
Advantages
The overall sensitivity of BTA testing ranges from 9.3% to as high as 89%, with higher sensitivity in higher-grade tumors [38,52,53,54,55,56]. BTA stat also has the advantage of being a point-of-care test.
Disadvantages
The BTA stat has low specificity (approximately 50%) among patients with urinary tract infections, urinary calculi, nephritis, cystitis, BPH, hematuria and proteinuria [55,57,58,59]. The low specificity in these conditions is secondary to the test’s ability to detect both complement factor H–related protein and complement factor H. Complement factor H is present in human serum at high concentrations and therefore BTA stat testing may be falsely positive in benign, hematuria-causing conditions [19].
Markers Under Investigation
A number of biomarkers are under investigation and have not yet received FDA approval. A selection of these biomarkers is described in Table 2.
Table 2. Biomarkers Under Investigation
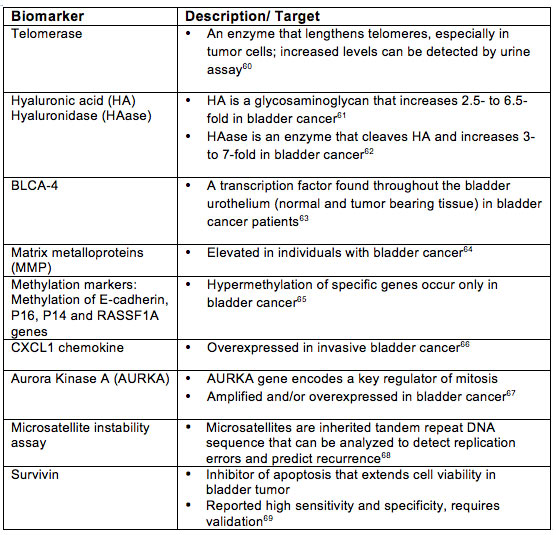
In summary, review of data on urine biomarkers for the detection of bladder cancer reveals that several have higher sensitivity than cytology, but most of these markers have lower specificity than cytology. An ideal biomarker must have both high sensitivity and high specificity. Unfortunately, none of these biomarkers meets this standard currently. Some biomarkers can be used to complement the present gold standard of cystoscopy and urine cytology, but none is ready to replace them.
Q4
UroToday: What is hexaminolevulinate-guided fluorescence cystoscopy? What is its role in the evaluation of bladder cancer?
The rationale for the use of photosensitizing drugs is based on the preferential accumulation of a photosensitizing compound in cancer cells. This compound emits fluorescence in the red part of the spectrum under blue-violet excitation or illumination and enhances detection of the tumor. The first prodrug used for this purpose was 5-aminolevulinic acid (5-ALA). Although 5-ALA itself has no photochemical activity, it induces the formation of endogenous photoactive porphyrins. Protoporphyrin IX is a photoactive derivative of ALA. Hexaminolevulinic acid (HAL) is a more potent ester of aminolevulinic acid that provides better selectivity, brighter fluorescence, and permits a shorter instillation time [70].
Hexaminolevulinate was approved the FDA on May 28, 2010, for the detection of NMIBC or suspected bladder cancer. Fluorescence-guided biopsy and resection have been confirmed as more sensitive than conventional procedures in detecting malignant tumor, particularly CIS [71,72,73].
Technique
The Cysview kit contains a 10 mL vial of 100 mg powder of hexaminolevulinate and a 50 mL vial of diluent [74]. The reconstituted solution is administered intravesically and retained for 1 hour. Standard WLC (Figure 2a) and blue light cystoscopy (Figure 2b) using a D-light cystoscope (Karl Storz, Germany) is then performed.
Figure 2a. Cystoscopic Examination With White Light
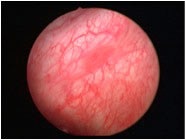
Figure 2b. Cystoscopic Examination With Blue Light

Images courtesy of Priv. Doz. Dr. med. Maximilian Burger Oberarzt, Klinik für Urologie der Universität Regensburg am Caritas- Krankenhaus St. Josef. Provided via GE Healthcare.
Hexaminolevulinate should not be used in patients with porphyria, gross hematuria, BCG immunotherapy or intravesical chemotherapy within the past 90 days, or a known hypersensitivity to HAL or ALA derivatives. Caution should be used in administering hexaminolevulinate to pregnant women and nursing mothers as there is no human or animal data available regarding its use in these populations. The safety and efficacy of hexaminolevulinate has not been established in pediatric patients [74].
Side effects of hexaminolevulinate are infrequent and most commonly minor in severity. The most common adverse effect was bladder spasm occurring in < 3% of patients and dysuria, hematuria, bladder pain, procedural pain, urinary retention and headache, all occurring in ≤ 2% patients. False fluorescence may occur due to inflammation, cystoscopic trauma, scar tissue or previous bladder biopsy [74].
Efficacy
Efficacy of PDD is evaluated by (1) detection of tumors and subsequent resection and (2) tumor recurrence. In a review of the literature, Witjes and colleagues concluded that HAL PDD provides considerable benefits versus WLC in the detection of NMIBC [75]. Furthermore, the benefits are most pronounced for CIS. The ability to detect CIS is approximately 28% greater with the addition of HAL PDD added to WLC compared to WLC alone [71]. Jocham and colleagues demonstrated the clinical benefit of an improved tumor detection rate. In their prospective, phase 3 multicenter study they found that PDD resulted in more complete treatment in 17% of patients (P < .0001) [76]. Mowatt and colleagues performed a systematic review of the effectiveness of PDD at the time of primary TURBT and noted that PDD enhances more complete resection rates and as a result prolongs recurrence-free survival compared with WLC [77]. In 3 trials evaluating tumor-free recurrence rates, PDD was associated with fewer recurrences, and the recurrence-free survival rate was 15.8% to 27% higher at 12 months and 12% to 15% higher at 24 months in the PDD groups than in the WLC-only groups [78,79,80].
Outpatient cystoscopy is typically performed with flexible cystoscopes. Data on the efficacy of PDD has been based on rigid cystoscopy. Loidl and colleagues performed a prospective controlled, within-patient comparison of flexible HAL cystoscopy with standard flexible cystoscopy, HAL rigid and standard white-light rigid cystoscopy. In the 45 patients studied 41 (91%) had exophytic tumors, of which 29 (95.1%) were detected by HAL flexible cystoscopy and 40 (97.5%) by HAL rigid cystoscopy. Seventeen (38%) patients had concomitant or CIS only; of these patients, CIS was identified by HAL flexible cystoscopy in 14 (82.3%), HAL rigid cystoscopy in 15 (88.2%), flexible WLC in 11 (64.7%), and rigid WLC in 13 (76.7%). Thus, HAL flexible cystoscopy was superior to WLC and comparable to HAL rigid cystoscopy in detecting papillary and flat lesions in bladder cancer patients [81].
Q5:
UroToday: What is the role of intravesical therapy after TURBT in the management of bladder cancer? What are current and emerging agents being used in intravesical therapy after TURBT?
Although TURBT is the gold standard for the initial diagnosis and treatment of NMIBC, intravesical therapy has become an integral component in the management of NMIBC [82].
Intravesical therapy is used to reduce and/or delay the risk for recurrence [83,84,85,86,87,88], prevent progression of disease, and as adjunctive therapy in Tis where diffuse tumor prevent complete tumor resection [89,90]. Most of the commonly used intravesical therapies for NMIBC can be categorized in 2 groups, immunomodulatory agents and chemotherapeutic agents, primarily based on their mechanism of action. Table 3 describes the mechanism of action and dosing for commonly used immunotherapeutic and chemotherapeutic agents.
Table 3. Intravesical Therapy

Selection of an agent for intravesical therapy is dictated by the risk for recurrence and progression in an individual patient. For tumors with a high risk for recurrence but low risk for progression (i.e., multiple recurrent Ta G1 tumors), either intravesical chemotherapy or BCG might be given [91]. For other tumors that have a high risk for recurrence and progression (i.e., multiple recurrent T1 G3 tumors), intravesical BCG with maintenance BCG therapy might be initiated.
Treatment guidelines have been published on risk stratification for the management of NMIBC [92,93,94,95,96]. The International Bladder Cancer Group (IBCG) developed treatment algorithms for the management of patients with low-, intermediate-, and high-risk NMIBC (Figures 3-5) [97].
Figure 3. Algorithm for the Treatment and Management of Low-Risk NMIBC

*Except in those with overt or suspected bladder wall perforation.
†Decision should be based on multifocality, size, grade, and stage.
Lamm DL. Oncology. 1995;9:947-952.
Figure 4. Algorithm for the Treatment and Management of Intermediate-Risk NMIBC
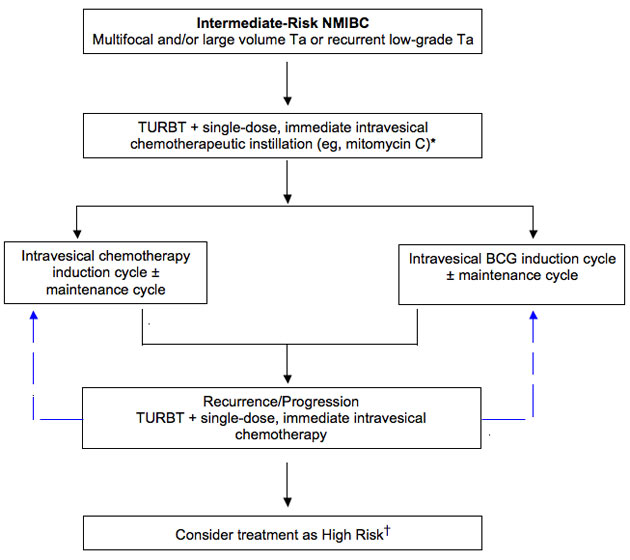
*Except in patients with overt or suspected bladder wall perforation.
†Decision should be based on grade, stage, size of tumor, and multifocality.
Lamm DL. Oncology. 1995;9:947-952.
Figure 3. Algorithm for the Treatment and Management of High-Risk NMIBC

Lamm DL. Oncology. 1995;9:947-952.
Immunotherapeutic Agents
BCG
BCG, a live, attenuated strain of mycobacterium bovis, is widely used as an intravesical immunotherapy against NMIBC since the 1970s. BCG is the first-line treatment for CIS and has been shown to be effective as prophylaxis to prevent recurrence following TURBT [98,99,100,101].
Initiation of intravesical BCG therapy is usually delayed for 2 to 3 weeks following TURBT to allow for healing of the urothelium and thereby decrease the risk for systemic side effects. Meta-analysis suggests that maintenance BCG therapy should be administered [92], but the optimal schedule and duration of therapy is not well-defined. The best available evidence, however, supports the use of the SWOG regimen comprising a 6-week induction cycle followed by a 3-week maintenance course at 3, 6, 12, 18, 24, 30, and 36 months, if tolerated by the patient [92,102].
Interferon
Recombinant interferon alpha-2b has been used as monotherapy and in combination with low-dose BCG therapy to treat patients with NMIBC [103,104,105,106]. Phase 2 trials have suggested durable response in both BCG-naïve and BCG-refractory patients, but long-term randomized trials have yet to be conducted to validate the results [92].
Chemotherapeutic Agents
Thiotepa
Thiotepa (triethylenethiophosphoramide) is an alkylating agent. Introduced in 1961, thiotepa is the oldest and one of the least expensive of the intravesical agents. The usual regimen consists of 6 to 8 weekly instillations followed by monthly instillations for 1 year [92]. Absorption though the urothelium may lead to systemic side effects—specifically myelosuppression—thus, monitoring of leukocyte and platelet count is needed before each instillation, and treatment should be delayed, if necessary.
Mitomycin C
Mitomycin C is an antibiotic that inhibits DNA synthesis. Myelosuppression and other systemic side effects are rare. Meta-analysis by the AUA guidelines panel have demonstrated a relative recurrence risk reduction of 17% with a single perioperative dose of mitomycin C in patients with NMIBC with both low- and high-risk features [85,92]. Perioperative mitomycin C should not be administered to patients with a known or suspected bladder perforation following TURBT.
Intercalating Agents (Doxorubicin, Epirubicin, and Valrubicin)
Doxorubicin is an anthracycline derivative. Absorption from the bladder and systemic toxicities are extremely rare following intravesical therapy.
Epirubicin is not currently available in the United States.
Valrubicin, a semisynthetic analogue of doxorubicin, is approved by the US FDA for intravesical therapy of BCG-refractory CIS of the bladder. Upon intravesical administration, valrubicin rapidly traverse cell membranes and accumulates in the cytoplasm, where it interferes with the incorporation of nucleoside into the nucleic acids, resulting in chromosomal damage and cell cycle arrest in G2 [107]. The metabolites of valrubicin also inhibit DNA synthesis. The response rate with intravesical valrubicin in patients with BCG-refractory CIS of the bladder is modest (18%) [108]. Thus, valrubicin is used in patients who refuse or are unfit for cystectomy.
Newer Approaches
Gemcitabine
The chemotherapeutic agent gemcitabine is an antimetabolite analog of the nucleoside pyrimidine. Intravesical gemcitabine has been shown to have activity in NMIBC [109,110,111,112]. A recent randomized prospective study comparing intravesical BCG versus gemcitabine in high-risk superficial bladder cancer demonstrated that gemcitabine is significantly inferior to BCG. At a median followup of 44 months, the recurrence rate in patients treated with BCG was 28.1%, compared to 53% in patients who received gemcitabine (P = .037) [113]. Gemcitabine may be useful in patients intolerant to or otherwise unable to receive BCG.
A phase 2 trial of intravesical gemcitabine in 30 patients with BCG refractory/intolerant disease who refused cystectomy, showed a 50% complete response (95% CI, 32, 68), and one year recurrence-free survival rate of patients with CR was 21% (95% CI, 0, 43) [111].
Docetaxel
Docetaxel is a microtubule, depolymerization inhibitor with antitumor activity. In a dose escalation study 18 patients were treated. Ten (56%) of 18 patients had no evidence of disease at their post-treatment cystoscopy and biopsy [114]. Intravesical docetaxel appeared to be safe and well tolerated. Further studies and long term follow-up is needed.
Other Emerging Agents
Other emerging agents include nab-paclitaxel, UrocidinTM, and apaziquone. Nab-paclitaxel is a novel agent in the taxane family that has been modified with the addition of albumin particles to form nanoparticles to increase solubility and facilitate drug delivery to tumor cells via biological interaction with albumin receptors that mediate drug transport across epithelial cells. In a phase 1 trial of 18 patients with BCG-refractory NMIBC using intravesical nab-paclitaxel, 5 patients (28%) had no evidence of disease at posttreatment cystoscopy [115].
Urocidin, a mycobacterial cell wall-DNA complex (MCC) formulation, that acts dually to stimulate immunity and has direct anticancer activity, is also being studied as a treatment for patients with bladder cancer. A recent Phase 3 trial demonstrated efficacy and safety of MCC in patients with NMIBC who were refractory to intravesical BCG therapy and at high risk of progression [116].
Apaziquone is another promising future therapy for the treatment of NMIBC. In a study that included 46 patients who had previously failed multiple therapies, apaziquone produced a 67% complete response and was well tolerated. Local side effects were comparable to side effects associated with other chemotherapy instillations [117].
Role of Intravesical Therapy in NMIBC
Role of immediate, postoperative, single-dose instillation of intravesical chemotherapy following TURBT.
A single, immediate instillation of intravesical mitomycin C following TURBT significantly reduces the risk for recurrence by 17% (95% CI, -28, -8%) when compared to TURBT alone [92].
Meta-analysis by the European Organization for the Research & Treatment of Cancer (EORTC) showed an absolute risk reduction of 12% with the use of a single, immediate post operative (within 24 hours) instillation of a chemotherapeutic agent following TURBT.85 No significant difference in efficacy among the chemotherapeutic agents (doxorubicin, epirubicin, mitomycin C) was noted.
European Association of Urology (EAU) guidelines recommend one immediate post operative instillation of chemotherapy in all patients after TUR of presumed NMIBC [96]. The American Urological Association (AUA) guidelines panel considered the immediate use of intravesical chemotherapy an option and not a standard because of potential cost issues, uncertainty of pathology, side effects and patient preference [92].
Immediate instillation of intravesical chemotherapy should not be done in the event of overt or suspected bladder perforation or excessive bleeding following TURBT. BCG can never be safely administered immediately after TURBT because of the risk for bacterial sepsis and death [118].
Role of intravesical therapy in the treatment of initial, small-volume, low-grade, Ta bladder cancer (low-risk disease)
Following complete TURBT, single-dose, immediate instillation of intravesical chemotherapy is recommended by the AUA and the EAU guidelines on NMIBC [92,96]. Meta-analysis and comparative study by the AUA guideline panel showed combination of TURBT and single-dose, immediate instillation of intravesical mitomycin C results in 17% (95% CI, -8, -28) fewer recurrences then TURBT alone when all patient risk-groups were considered [92]. A meta-analysis by the EAU guideline panel demonstrated that one immediate instillation of intravesical chemotherapy after TUR decreased the percentage of patients with recurrence by 12% (from 48.4% to 36.7%).96 The benefit was confirmed in both single and multiple tumors [85]. There is no evidence that multiple adjuvant instillations of either BCG or chemotherapy have additional benefits in patients at initial diagnosis of Ta Grade 1 bladder cancer [92].
Role of intravesical therapy in the treatment of patients with multifocal and/or large-volume, low-grade Ta or recurrent low-grade Ta bladder cancer (Intermediate-risk group):
Intermediate risk group patients should be treated with TURBT and immediate instillation of intravesical chemotherapy [85]. The AUA guideline panel recommends the use of induction course of intravesical BCG or mitomycin C in this group of patients with the goal of preventing or delaying recurrence [92]. In intermediate-risk patients, recurrences were reduced by 24% (95% CI, 3, 47) with the combination of TURBT and BCG induction only and by 3% (95% CI, -10, 16) with TURBT and mitomycin C induction compared with TURBT alone [92].
Role of maintenance intravesical therapy in intermediate-risk-group patients
Meta-analysis by the AUA guideline panel of randomized controlled trials between 1990 and 2006 demonstrated that compared to TURBT alone, recurrences are decreased by 31% (95% CI, 18, 42) with TURBT and BCG therapy (induction plus maintenance) and by 18% (95% CI, 6, 30) with TURBT and mitomycin C induction plus maintenance regimen [92].
The optimal maintenance schedule and duration has yet to be determined. However, the best available evidence supports the use of SWOG regimen of a 6-week induction course of BCG followed by a 3-week maintenance course at 3, 6, 12, 18, 24, 30, and 36 month if tolerated by the patient [92,119].
For intermediate-risk group patients, EAU guideline recommends the use of either BCG or mitomycin C maintenance regimen. The AUA guidelines panel advised that maintenance regimen in this group of patients is an option that should be discussed with the patient [92].
Role of intravesical therapy in the treatment of patients with an initial diagnosis of high-grade Ta, T1, and/or Tis bladder cancer (high-risk disease)
For patients with lamina propria invasion but without muscular propria in the specimen, repeat resection should be performed prior to intravesical therapy.92 Data suggest that 20% to 40% will have either residual tumor and/or unrecognized muscle-invasive disease [120,121,122].
High-grade T1 lesions recur in more than 80% of cases and progress in 50% of patients within 3 years [118]. The goal of treatment in this group of patients is to reduce both the risk for recurrence and progression.
Both the AUA and the EAU guidelines recommend the use of induction course of BCG followed by maintenance regimen.92,96 According to AUA, cystectomy is considered an option for initial therapy in select patients with high-grade Ta, T1 and/or CIS because of the risk of understaging muscle-invasive disease initially or progression to muscle-invasive disease [92]. According to the EAU, immediate radical cystectomy may be offered to the highest-risk patients, such as those with multiple recurrent tumors, high-grade T1 tumors, or high-grade tumors with CIS [96].
Role of BCG in the treatment CIS
A meta-analysis revealed a 68% complete response rate with BCG and 49% complete response rate with chemotherapy in patients with CIS. In the complete responders, 68% of patients treated with BCG remained disease-free as compared to 47% of patients receiving chemotherapy based on a median followup of 3.75 years. The overall disease-free rates were 51% and 27% [91,123].
The AUA guideline for the management of NMIBC and EAU guidelines on the diagnosis and treatment of urothelial carcinoma in situ recommends the use of induction course of BCG followed by maintenance cycle for CIS. BCG intravesical therapy is also approved by the FDA for use in CIS [92,123].
Treatment of patients with high-grade, Ta, T1, and/or CIS bladder cancer that has recurred after prior intravesical therapy
The standard treatment for patients with lamina propria invasion (T1) but without muscularis propria in the specimen, is repeat resection prior to further intravesical therapy. Cystectomy should be considered as a therapeutic alternative for these patients. The high likelihood of intravesical treatment failure and adverse consequence of delaying cystectomy make cystectomy the preferred treatment for these patients [92].
Further intravesical therapy is considered an option by the AUA guideline in these patients. There is some evidence that select patients will respond to second induction regimens, particularly with BCG [96,124,125]. Intravesical valrubicin therapy is approved by the FDA in BCG refractory CIS patients has a reported 18% disease-free rate at 6 months [108]. Repeat intravesical therapy may be appropriate in patients who develop a late recurrence after previous complete response to an intravesical agent.
Concluding Remarks
Bladder cancer is a common genitourinary malignancy with significant impact on quality of life, survival and economics. Early diagnosis and treatment remain the goal. Recent advances in urine markers, diagnostic techniques and intravesical therapies enhance the evaluation and treatment of this condition. An understanding of the advantages and limitations of newer urine biomarkers is critical to identifying the roles they may play in select patients. Furthermore, an understanding of the role of blue light cystoscopy with hexaminolevulinate plays in the detection of bladder cancer will help improve detection and optimize endoscopic treatment of bladder cancer. Lastly, optimal use of current intravesical therapy after TURBT may serve to decrease the risk of recurrent/progressive bladder cancer.
References
- American Cancer Society. Cancer Facts & Figures 2011. Atlanta, GA: American Cancer Society; 2011.
- National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Web site. SEER Stat Fact Sheets: Bladder. http://seer.cancer.gov/statfacts/html/urinb.html. Accessed July 12, 2011.
- Miller BA, Kolonel LN, Bernstein L, et al, eds. Racial/Ethnic Patterns of Cancer in the United States 1988-1992, National Cancer Institute. NIH Pub. No. 96-4104. Bethesda, MD, 1996. http://seer.cancer.gov/publications/ethnicity/. Accessed July 12, 2011.
- Sievert KD, Amend B, Nagele U, et al. Economics of bladder cancer: what are the benefits and costs? World J Urol. 2009;27(3):295-300.
- Brown ML, Riley GF, Schussler N, et al. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV-104-IV-117.
- Avritscher EB, Cooksley CD, Grossman HB, et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology. 2006;68:549-553.
- Sangar VK, Ragavan N, Matanhelia SS, Watsom MW, Blades RA. The economic consequences of prostate and bladder cancer in the UK. BJU Int. 2005;95:59-63.
- Stenzl A, Roessler W, Fradet Y, et al. Hexvix fluorescence cystoscopy improves detection and resection of papillary bladder cancer and reduces early recurrence: a multicentre, prospective, randomized study (abstract 1010). Poster presented at: 24th European Association of Urology (EAU) Congress; March 17–21, 2009; Stockholm, Sweden.
- Stenzl A, Burger M, Fradet Y, et al. Hexaminolevulinate guided fluorescence cystoscopy reduces recurrence in patients with nonmuscle invasive bladder cancer. J Urol. 2010;184:1907-1914.
- Daniltchenko DI, Riedl CR, Sachs MD, et al. Long-term benefit of 5-aminolevulinic acid fluorescence assisted transurethral resection of superficial bladder cancer: 5-year results of a prospective randomized study. J Urol. 2005;174:2129-2133.
- Malmstrom PU, Hedelin H. Potential cost savings through the use of fluorescence cystoscopy in superficial bladder cancer: development of an economic model. Urology Suppl. 2006;68:40-41.
- Burger M, Zaak D, Stief CG, et al. Photodynamic diagnostics and noninvasive bladder cancer: is it cost-effective in long-term application? A Germany-based cost analysis. Eur Urol. 2007;52:142-147.
- Jichlinski P, Leisinger HJ. Fluorescence cystoscopy in the management of bladder cancer: a help for the urologist! Urol Int. 2005;74:97-101.
- Frimberger D, Zaak D, Hofstetter A. Endoscopic fluorescence diagnosis and laser treatment of transition cell carcinoma of the bladder. Semin Urol Oncol. 2000;18:264-272.
- Hudson MA, Herr HW. Carcinoma in situ of the bladder. J Urol. 1995;153:564-572.
- Iczkowski KA, Katz G, Cascione CJ. Postoperative bladder washing cytology after transurethral resection: can it predict the recurrence of urothelial carcinoma? Acta Cytol. 2004;48:380-384.
- Badalament RA, Kimmel M, Gay H, et al. The sensitivity of flow cytometry compared with conventional cytology in the detection of superficial bladder carcinoma. Cancer. 1987;59:2078-2085.
- Burchardt M, Burchardt T, Shabsigh A, De La Talle A, Benson ML, Sawczuk I. Current concept in biomarker technology for bladder cancers. Clin Chem. 2000;46:595-605.
- Konety BR, Getzenberg RH. Urine based markers of urological malignancy. J Urol. 2001;165:600-611.
- Giannopoulos A, Manousakas T, Mitropoulos D, et al. Comparative evaluation of the BTAstat test, NMP22, and voided urine cytology in the detection of primary and recurrent bladder tumors. Urology. 2000;55:871-875.
- Keesee Sk, Brigman JV, Thill G, Wu YJ. Utilization of nuclear matrix protein for cancer diagnosis. Crit Rev Eukaryout Gene Expr. 1996;6:189-214.
- Lotan Y, Roehrban CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analysis. Urology. 2003;61:109-118.
- Nguyen CT, Jones JS. Defining the role of NMP 22 in bladder cancer surveillance. World J Urol. 2008;26:51-58.
- Grossman HB, Messing E, Katz G, Stein B, Kassabian V, Shen Y. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295:299-305.
- Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005;293:810-816.
- Tritschler S, Scharf S, Karl A, et al. Validation of the diagnostic value of NMP 22 bladder check test as a marker for bladder cancer by photodynamic diagnosis. Eur Urol. 2007;51:403-407; discussion 407-408.
- Moonen PM, Kiemeney LA, Witjer JA. Urinary NMP 22 bladder check test in the diagnosis of superficial bladder cancer. Eur Urol. 2005;48:951-956; discussion 956.
- Kumar A, Kumar R, Gupta NP. Comparison of NMP 22 Bladder check test and urine cytology for the detection of recurrent bladder cancer. Jpn J Clin Oncol. 2006;36:172-175.
- Sharma S, Zippe CD, Pandrangi L, Nelson D, Agarwal A. Exclusion criteria enhance the specificity and positive predictive value of NMP 22 and BTA stat. J Urol. 1999;162:53-57.
- Sullivan PS, Chan JB, Levin MR, Rao J. Urine cytology and adjunct markers for detection and surveillance of bladder cancer. Am J Trans Res. 2010;2:412-440.
- Halling KC, King W, Sokolova IA, et al. A comparison of cytology and Fluorescence in situ hybridization for the diction of urothelial carcinoma. J Urol. 2000;64:1768-1775.
- Varella-Garcia M, Akduman B, Sunpaweravong P, Di Maria MV, Crawford ED. The UroVysion fluorescence in situ hybridization assay is an effective tool for monitoring recurrence of bladder cancer. Urol Oncol. 2004;22:16-19.
- Friedrich MG, Toma MI, Hellstern A, et al. Comparison of multitarget fluorescence in situ hybridization in urine with other noninvasive tests for detecting bladder cancer. BJU Int. 2003;92:911-914.
- Sarosdy MD, Schellhammen P, Bokinsky G, et al. Clinical evaluation of multi-target fluorescent in situ hybridization assay for detection of bladder cancer. J Urol. 2002;168:1950-1954.
- Dalquen P, Kleiber B, Grilli B, Herzog M, Bubendorf L, Oberholzer M. DNA image cytometry and fluorescence in situ hybridization for noninvasive detection of urothelial tumors in voided urine. Cancer. 2002;96:374-379.
- Laudadio J, Keane TE, Reeves HM, et al. Fluorescence in situ hybridization for detecting transitional cell carcinoma: implication for clinical practice. BJU Int. 2005;96:1280-1285.
- Bergman J, Reznichek RC, Rajfer J. Surveillance of patients with bladder carcinoma using fluorescent in situ hybridization on bladder washings. BJU Int. 2008;19:26-29.
- Halling KC, King W, Sokolova IA, et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J Urol. 2002;167:2001-2006.
- Sokolova IA, Halling KC, Jenkin RB, et al. The development of multitarget multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn. 2000;2:116-123.
- Skacel M, Fahmy M, Brainard JA, et al. Multitarget fluorescence in situ hybridization assay detects its transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J Urol. 2003;169:2101-2105.
- Gudjonsson S, Isfoss BL, Hansson K, et al. The value of the UroVysion assay for surveillance of non-muscle invasive bladder cancer. Eur Urol. 2008;54:402-408.
- Moonen PM, Merkx GF, Peelen P, Karthaus HF, Smeets DF, Witjes JA. UroVysion compared with cytology and quantitative cytology in the surveillance of non-muscle invasive bladder cancer. Eur Urol. 2007;51:1275-1280; discussion 1280.
- Junker K, Fritsch T, Hartmann A, Schultze W, Schubert J. Multicolor fluorescence in situ hybridization (M-FISH) on cells from urine for the detection of bladder cancer. Cytogent Genome Res. 2006;114:279-283.
- Lodde M, Mian C, Negri G, et al. Role of uCyt+ in the detection and surveillance of urothelial carcinoma. Urology. 2003;61:243-247.
- Pfister C, Chautard D, Devone M, et al. Immunocyt test improves the diagnostic accuracy of urinary cytology: Results of a French multicenter study. J Urol. 2003;169:921-924.
- Fell G, Zumbragel A, Paulgen-Nelde HJ, et al. Accuracy of the Immunocyt assay in the diagnosis of transitional cell carcinoma of the urinary bladder. Anticancer Res. 2003;23:963-967.
- Tetu B, Tiguert R, Harel F. Fradet Y. Immunocyt/uCyt+ improves the sensitivity of urine cytology in patients followed for urothelial carcinoma. Mod Pathol. 2005;18:83-89.
- Lodde M, Mian C, Comploj E, et al. Ucyt+ test: Alternative to cystoscopy for less invasive follow-up of patients with low risk of urothelial carcinoma. Urology. 2006;67:950-954.
- Mian C, Maier K, Comploj E, et al. uCyt+/ImmunoCyt in the detection of recurrent urothelial carcinoma: an update on 1991 analyses. Cancer. 2006;108:60-65.
- Messing EM, Teot L, Korman H, et al. Performance of urine test in patients monitored for recurrence of bladder cancer: A multicenter study in the United States. J Urol. 2005;174:1238-1241.
- Lokeshwar VB, Habuchi T, Grossman HB, et al. Bladder tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology. 2005;66:35-63.
- Gutierrez Banos JL, del Henar Rebollo Rodrigo M, Antolín Juarez FM, Garcia BM. Usefulness of the BTA STAT Test for the diagnosis of bladder cancer. Urology. 2001;57:685-689.
- Raitanen MP, Marttila T, Kaasinen E, Rintala E, Aine R, Tammela TL. Sensitivity of human complement factor H related protein (BTA stat) test and voided urine cytology in the diagnosis of bladder cancer. J Urol. 2000;163:1689-1692.
- Schroeder GL, Lorenzo-Gomez MF, Hautmann SH, et al. Side by side comparison of cytology and biomarkers for bladder cancer detection. J Urol. 2004;172:1123-1126.
- Lokeshwar VB, Schroeder GL, Selzer MG, et al. Bladder tumor markers for monitoring recurrence and screening comparison of hyaluronic acid-hyaluronidase and BTA-Stat Tests. Cancer. 2002;95:61-72.
- Raitanen MP. The role of BTA stat Test in follow-up of patients with bladder cancer: results from FinnBladder studies. World J Urol. 2008;26:45-50.
- Heicappell R, Muller M, Fimmers R, Miller K. Qualitative determination of urinary human complement factor H-related protein (hcfHrp) in patients with bladder cancer, healthy controls, and patients with benign urologic disease. Urol Int. 2000;65:181-184.
- Serretta V, Pomara G, Rizzo I, Esposito E. Urinary BTA-stat, BTA-trak and NMP22 in surveillance after TUR of recurrent superficial transitional cell carcinoma of the bladder. Eur Urol. 2000;38:419-425.
- Oge O, Kozaci D, Gemalmaz H. The BTA stat test is nonspecific for hematuria: an experimental hematuria model. J Urol. 2002;167:1318-1319; discussion 1319-1320.
- Bennett A. Telomerase and other novel approaches to bladder cancer detection. Clin Lab Sci. 2008;21:185-190.
- Lokeshwar VB, Block NL. HA-HAase urine test: a sensitive and specific method for detecting bladder cancer and evaluating its grade. Urol Clin North Am. 2000;27:53-61.
- Pham HT, Block NL, Lokeshwar VB. Tumor-derived hyaluronidase: a diagnostic urine marker for high-grade bladder cancer. Cancer Res. 1997;57:778-783.
- Konety BR, Nguyen TS, Dhir R, et al. Detection of bladder cancer using a novel nuclear matrix protein, BLCA-4. Clin Cancer Res. 2000;6:2618-2625.
- Roy R, Loui G, Loughlin KR, et al. Tumor specific urinary matrix metalloproteinase fingerprinting identification of high molecular weight urinary matrix metalloproteinase species. Clin Cancer Res. 2008;14:6610-6617.
- Lin HH, Ke HL, Huang SP, Wu WJ, Chen YK, Change LL. Increase sensitivity in detecting superficial, low grade bladder cancer by combination analysis of hypermethylation of E-cadherin, p16, p14, RASSF1A genes in urine. Urol Oncol. 2010;28:597-602.
- Kawanishi H, Matsui Y, Ito M, et al. Secreted CXCL1 is a potential mediator and marker of the tumor invasion of bladder cancer. Clin Cancer Res. 2008;14:2579-2581.
- Park HS, Park WS, Bondaruk J, et al. Quantitation of Aurora Kinase a gene copy number in urine sediments and bladder cancer detection. J Natl Cancer Inst. 2008;100:1401-1411.
- Brentnall TA. Microsatellite instability. Shifting concepts in tumorigenesis. Am J Pathol. 1995;147:561-563.
- Smith SD, Wheeler MA, Plescia J, Colberg JW, Weiss RM, Altieri DC. Urine detection of survivin and diagnosis of bladder cancer. JAMA. 2001;285:324-328.
- Marti A, Jichlinski P, Lange N, Ballini JP, Guillou L, Leisinger HJ, Kucera P. Comparison of aminolevulinic acid and hexylester aminolevulinate induced protoporphyrin IX distribution in human bladder cancer. J Urol. 2003;170:428–432.
- Schmidbauer J, Witjes F, Schmeller N, et al; Hexvix PCB301/01 Study Group. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004; 171:135-138.
- Jichlinski P, Guillou L, Karlsen SJ, et al. Hexyl aminolevulinate fluorescence cystoscopy: a new diagnostic tool for photodiagnosis of superficial bladder cancer—a multicenter study. J Urol. 2003;170:226-9.
- Hungerhuber E, Stepp H, Kriegmair M, Stief Ch, Hofstetter A, Hartmann A, Knuechel R, Karl A, Tritschler S, Zaak D. Seven years´ experience with 5-aminolevulinic acid in detection of transitional cell carcinoma of the bladder. Urology. 2007;69:260-264.
- GE Healthcare Web Site. Cysview™ hexaminolevulinate HCl. www.cysview.net/. Accessed July 25, 2011.
- Witjes JA, Redorta JP, Jacqmin D, et al. Hexaminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non–muscle-invasive bladder cancer: Review of the evidence and recommendations. Eur Urol. 2010;57:607-614.
- Jocham D, Witjes F, Wagner S, et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: a prospective, phase III multicenter study. J Urol. 2003;170:226-229.
- Mowatt G, N’Dow J, Vale L, et al. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: Systematic review and meta-analysis. Int J Technol Assess Health Care. 2011;27:3-10.
- Babjuk M, Soukup V, Petrik R, Jirsa M, Dvoracek J. 5-Aminolevulinic acid-induced fluorescence cystsocopy during transurethral resection reduces the risk of recurrence in stage Ta/T1 bladder cancer. BJU Int. 2005;96:798-802.
- Filbeck T, Pichlmeier U, Knuechel R, Wieland WF, Roessler W. Clinically relevant improvement of recurrence-free survival with 5-aminolevulinic acid induced fluorescence diagnosis in patients with superficial bladder tumors. J Urol. 2002;168:67-71.
- Riedl CR, Daniltchenko D, Koenig F, Simak R, Loening SA, Pflueger LH. Fluorescence endoscopy with 5-aminolevulinic acid reduces early recurrence rate in superficial bladder cancer. J Urol. 2001;165:1121-1123.
- Loidl W, Schmidbauer J, Susani M, Marberger M. Flexible cystoscopy assisted by hexaminolevulinate induced fluorescence: a new approach for bladder cancer detection and surveillance? Eur Urol. 2005;47:323-326.
- Crawford ED. Intravesical therapy for superficial cancer: need for more options. J Clin Oncol. 2002;20:3185-3186.
- Bouffioux C, Kurth KH, Bono A, Oosterlinck W, Kruger CB, DePauw M, et al. Intravesical adjuvant chemotherapy for superficial transitional cell bladder carcinoma: results of 2 European Organization for Research and Treatment of Cancer randomized trials with mitomycin C and doxorubicin comparing early versus delayed instillation and short-term versus long-term treatment. European Organization for Research and Treatment of Cancer Genitourinary Group. J Urol. 1995;153:934.
- Tolley DA, Parmar MK, Grigor KM, Lallemand G, Benyon LL, Fellows J, et al. The effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: a further report with 7 years of follow up. J Urol. 1996;155:1233-1238.
- Sylvester RJ, Oosterlinck W, van der Meijen AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186-2190.
- Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guérin immunotherapy for recent TA T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000; 163:1124-1129.
- Huncharek M, Geschwind JF, Witherspoon B, McGarry R, Adcock D. Intravesical chemotherapy prophylaxis in primary superficial bladder cancer: a meta-analysis of 3703 patients from 11 randomized trials. J Clin Epidemiol. 2000;53:676-680.
- Huncharek M, McGarry R, Kupelnick B. Impact of intravesical chemotherapy on recurrence rate of recurrent superficial transitional cell carcinoma of the bladder: results of a meta-analysis. Anticancer Res. 2001;21:765-769.
- Shelley MD, Kynaston H, Court J, Wilt TJ, Coles B, Burgon K, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001;88:209-216.
- Sylvester RJ, van de Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guérin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964-1970.
- Sylvester RJ, van der Meijden A, Witjes JA, et al. High grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology. 2005;66:90-107.
- Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of non-muscle invasive bladder cancer (stages Ta, T1 and Tis): 2007 update. J Urol. 2007;178:2314-2330.
- Soloway MS, editor. International consultation on bladder tumours. Urology. 2005;66:1-125. [sent inquiry to Dr. Choudhury, PKS, 7/27]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Bladder Cancer Including Upper Tract Tumors and Urothelial Carcinoma of the Prostate. Version 2.2011. Fort Washington, PA: NCCN; 2011.
- American Urological Association. Guidelines for the management of non-muscle invasive balder cancer (stages Ta, T1 and Tis): 2007 update. Linthicum, MD: American Urological Association; 2007.
- Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou J. European Association of Urology guidelines on TaT1 (non-muscle invasive) bladder cancer. Update March 2008. Arnhem, Netherlands: European Association of Urology; 2008. http://www.uroweb.org/fileadmin/user_upload/Guidelines/TaT1%20Bladder%20Cancer.pdf.
- Lamm D, Colombel M, Persad R, et al. Clinical practice recommendation for the management of non-muscle invasive bladder cancer. Eur Urol Suppl. 2008;7:651-666.
- Lamm DL. BCG immunotherapy for transitional-cell carcinoma in situ of the bladder. Oncology. 1995;9:947-952.
- Cookson MS and Sarosdy MF. Management of stage T1 superficial bladder cancer with intravesical bacillus Calmette-Guérin therapy. J Urol. 1992;148:797-801.
- Coplen DE, Marcus MD, Myers JA, Ratliff TL, Catalona WJ. Long-term followup of patients treated with 1 or 2, 6-week courses of intravesical bacillus Calmette-Guérin: analysis of possible predictors of response free of tumor. J Urol. 1990;144:652-657.
- De Jager R, Guinan P, Lamm D, et al. Long-term complete remission in bladder carcinoma in situ with intravesical TICE bacillus Calmette-Guérin. Overview analysis of six phase II clinical trials. Urology. 1991;38:507-513.
- Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guérin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90-95.
- Stricker P, Pryor K, Nicholson T, et al. Bacillus Calmette-Guérin plus intravesical interferon alpha-2b in patients with superficial bladder cancer. Urology. 1996;48:957-961.
- Mohanty NK, Malhotra V, Nayak RL, Arora RP. Combined low-dose intravesical immunotherapy (BCG + interferon alpha-2b) in the management of superficial transitional cell carcinoma of the urinary bladder: a five-year follow-up. J Chemother. 2002;14:194-197.
- Lamm JS, Benson MC, O’Donnell MA, et al. Bacillus Calmette-Guérin plus interferon-alpha2B intravesical therapy maintains an extended treatment plan for superficial bladder cancer with minimal toxicity. Urol Oncol. 2003;21:354-360.
- Joudi FN, Smith BJ, O’Donnell MA and National BCG-Interferon Phase 2 Investigator Group: Final results from a national multicenter phase II trial of combination bacillus Calmette-Guérin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol. 2006;24:344-348.
- Grossman BH, O’Donnell MA, Cookson MS, Greenberg RE, Keane TE. Bacillus Calmette-Guérin failures and beyond: contemporary management of non-muscle-invasive bladder cancer. Rev Urol. 2008;10:281-289.
- Steinberg G, Bahnson R, Brosman S, et al. Efficacy and safety of valrubicin for the treatment of bacillus Calmette-Guérin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol. 2000;163:761-767.
- Bartoletti R, Cai T, Gacci M, et al. Intravesical gemcitabine therapy for superficial transitional cell carcinoma: results of a Phase II prospective multicenter study. Urology. 2005;66:726-731.
- Mattioli F, Curotto A, Manfredi V, et al. Intravesical gemcitabine in superficial bladder cancer: a phase II safety, efficacy and pharmacokinetic study. Anticancer Res. 2005;25:2493-2496.
- Dalbagni G, Russo P, Bochner B, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guérin refractory transitional cell carcinoma of the bladder. J Clin Oncol. 2006;24:2729-2734.
- Hendricksen K, Witjes JA. Intravesical gemcitabine: an update of clinical results. Curr Opin Urol. 2006;16:361-366.
- Porena M, Del Zingaro M, Lazzeri M, et al. Bacillus Calmette-Guérin versus gemitabine for intravesical therapy in high-risk superficial bladder cancer: A randomized prospective study. Urol Int. 2010;84:23-27.
- McKiernan JM, Masson P, Murphy AM, et al. Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. J Clin Oncol. 2006;24:3075-3080.
- McKiernan JM, Barlow LJ, Laudano MA, Mann MJ, Petrylak DP, Benson MC. A phase I trial of intravesical nanoparticle albumin-bound paclitaxel in the treatment of bacillus Calmette-Guérin refractory non-muscle invasive bladder cancer. J Urol. 2011;186:448-451.
- Morales A, Herr H, Kamat A, et al. Phase 3 study to evaluate the efficacy and safety of mycobacterial cell wall-DNA complex in the treatment of patients with non-muscle invasive bladder cancer at high risk of progression and who are refractory to BCG. Podium abstract presented at: American Urological Association Annual Meeting; May 14-19, 2011; Washington, DC. Abstract 1650.
- van der Heijden AG, Moonen PM, Cornel EB, et al. Phase II marker lesion study with intravesical instillation of apaziquone for superficial bladder cancer: toxicity and marker response. J Urol. 2006;176(4 pt 1):1349-1353.
- Jones SJ, Campbell SC. Chapter 76. Non-muscle invasive bladder cancer (Ta, T1 and CIS). In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 9th ed. Philadelphia, Pa: Saunders Elsevier; 2007.
- Bohle A, Bock PR. Intravesical bacille Calmette-Guérin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63:682-686.
- Dutta SC, Smith JA Jr, Shappell SB, Coffey CS, Chang SS, Cookson MS. Clinical under staging of high risk non-muscle invasive urothelial carcinoma treated with radical cystectomy. J Urol. 2001;166:490-493.
- Schwaibold HE, Sivalingam S, May F, Hartung R. The value of second transurethral resection for T1 bladder cancer. BJU Int. 2006;97:1199-1201.
- Brauers A, Buettner R, Jakse G. Second resection and prognosis of primary high risk superficial bladder cancer: is cystectomy often too early? J Urol. 2001;165:808-810.
- van der Meijden AP, Sylvester R, Oosterlinck W, et al; for the EAU working party on non-muscle invasive bladder cancer. EAU guidelines on the diagnosis and treatment of carcinoma in situ. Eur Urol. 2005;48:363-371.
- de Reijke TM, Kurth KH, Sylvester RJ, et al. Bacillus Calmette-Guérin versus epirubicin for primary, secondary or concurrent carcinoma of the bladder: results of a European Organization for the Research and Treatment of Cancer – Genito-Urinary Group Phase III Trial (30906). J Urol. 2005;173:405-409.
- Catalona WJ, Hudson MA, Gillen DP, Andriole GL, Ratliff TL. Risks and benefits of repeated courses of intravesical bacillus Calmette-Guérin therapy for superficial bladder cancer. J Urol. 1987;137:220-224.
To complete the posttest and evaluation for this activity and receive CME credit, click here


