(UroToday.com) Novel androgen receptor targeted agents (ARTA) are effective in improving patient outcomes across the spectrum of prostate cancer disease states. Resistance to ARTAs eventually develops in advanced prostate cancer, and a broader understanding of the mechanisms promoting this resistance is required. Mutations within the androgen receptor (AR) gene itself have been linked to anti-androgen resistance.
Indeed, two publications in 2013 linked the F876L mutation to enzalutamide resistance.1,2 To more broadly explore AR alterations that may confer resistance to ARTAs, Dr. Zeynep Zengin and colleagues utilized the PROMISE consortium database, a multi-institutional retrospective clinicogenomic database of advanced prostate cancer patients who have had tissue or blood based genomic sequencing using CLIA-certified commercial platforms.
The authors identified 854 patients who received an ARTA and had either tissue (n = 600) or blood (n = 335) based genomic testing. The study workflow is shown below.
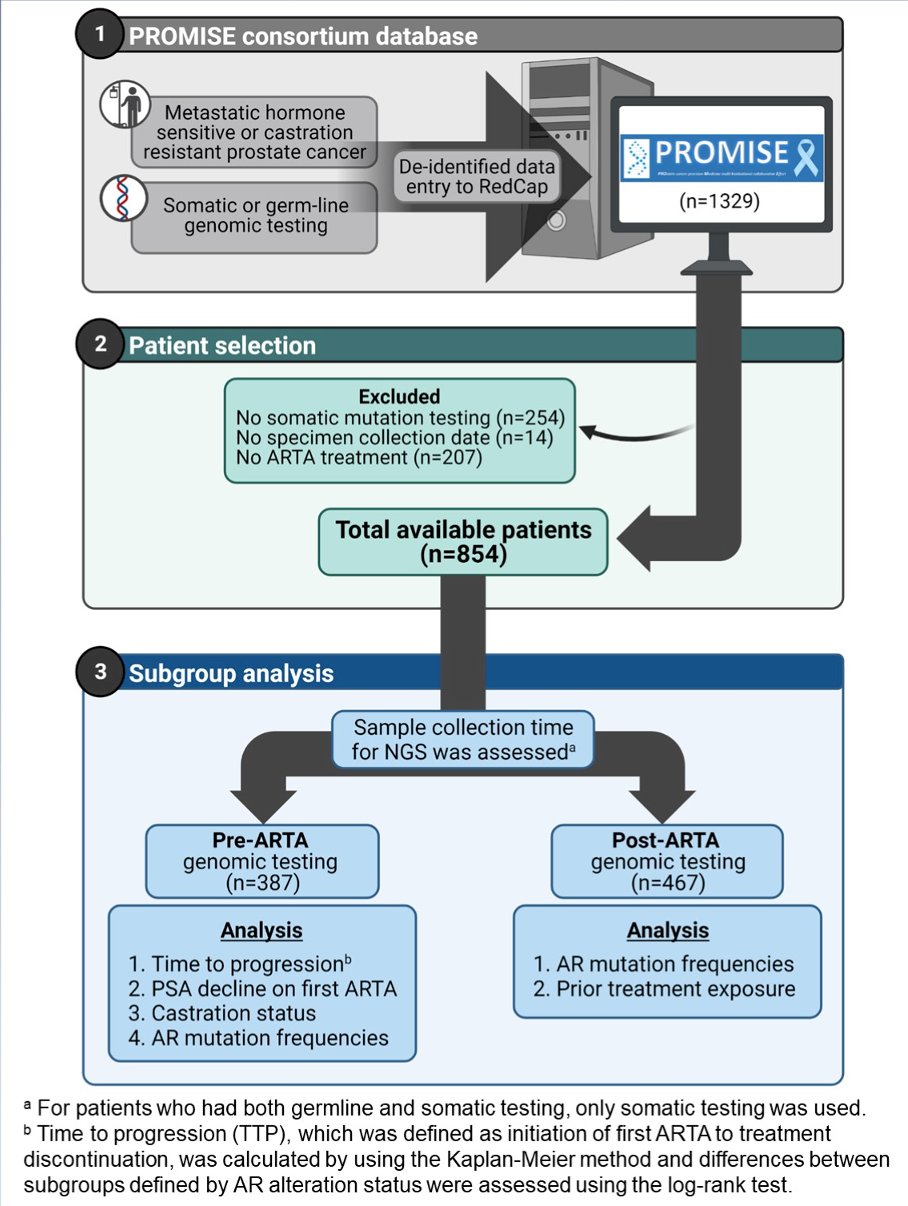
Patient characteristics from this cohort are shown in the following table.
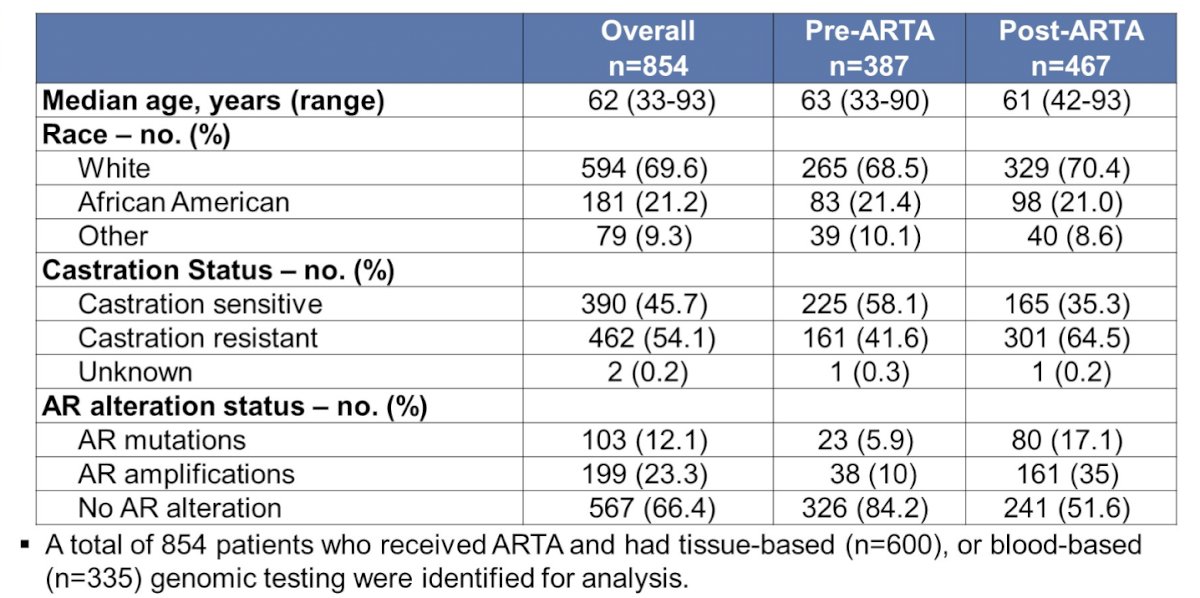
Of 387 patients with genomic sequencing prior to ARTA initiation, 23 had an AR mutation and 38 had an AR amplification. The most common pre-ARTA AR mutation was H875Y (6 patients), with 5 patients having L702H, T878A, and W742C, respectively. The presence of a pre-ARTA AR mutation was statistically significantly associated with a shorter median time to progression, whereas the presence of AR amplification was associated with a longer median time to progression on therapy.
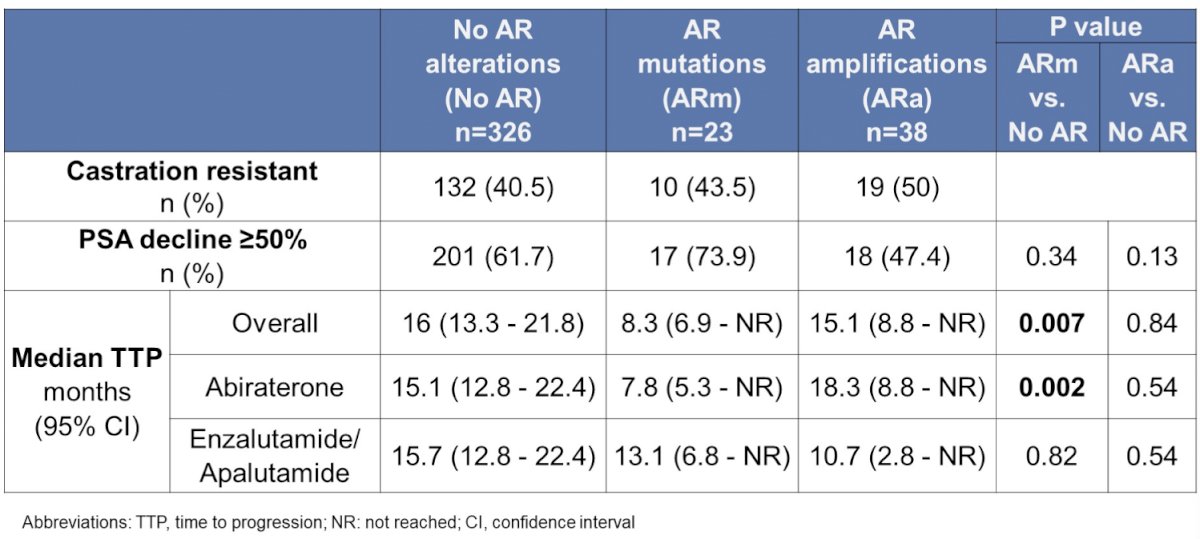
Of 467 patients who had genomic sequencing after ARTA initiation, 80 had AR mutations and 161 had AR amplification. Of the patients that had pre- and post-ARTA sequencing, a subset of 15 out of 52 patients developed a new AR mutation with treatment. This study suggests that pre-treatment AR mutation screening should be considered prior to potential ARTA selection in mCRPC.
Presented by: Zeynep Busra Zengin, MD, Postdoctoral Fellow, City of Hope Comprehensive Cancer Center, Duarte, CA
Written by: Alok K. Tewari, MD, PhD, medical oncologist at Dana-Farber Cancer Institute, @aloktewar on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday, Feb 17 – Saturday, Feb 19, 2022
References:
- Balbas MD, Evans MJ, Hosfield DJ, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife. 2013;2:e00499
- Korpal M, Korn JM, Gao X, et al. An F876L Mutation in Androgen Receptor Confers Genetic and Phenotypic Resistance to MDV3100 (Enzalutamide). Cancer Discov. 2013;3(9):1030-1043.


