(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Poster Session A focussed on the care of patients with prostate cancer. Dr. Herrmann presented a poster assessing [177Lu]Lu-PSMA-617 (177Lu-PSMA-617) dosimetry among patients treated on the phase 3 VISION trial (NCT03511664).
VISION demonstrated that 177Lu-PSMA-617 plus protocol-permitted standard of care (SOC) significantly improved overall survival and radiographic progression-free survival of patients with metastatic castration-resistant prostate cancer (mCRPC), compared with SOC alone. Further, while there was a higher incidence of adverse events (AEs) of grade 3 or above for patients receiving 177Lu-PSMA-617,
this did not adversely affect the quality of life. In the poster presented at this meeting, Herrmann provided results of a dosimetry sub-study aimed at quantifying the absorbed dose of 177Lu-PSMA-617 in organs at risk of radiotoxicity.
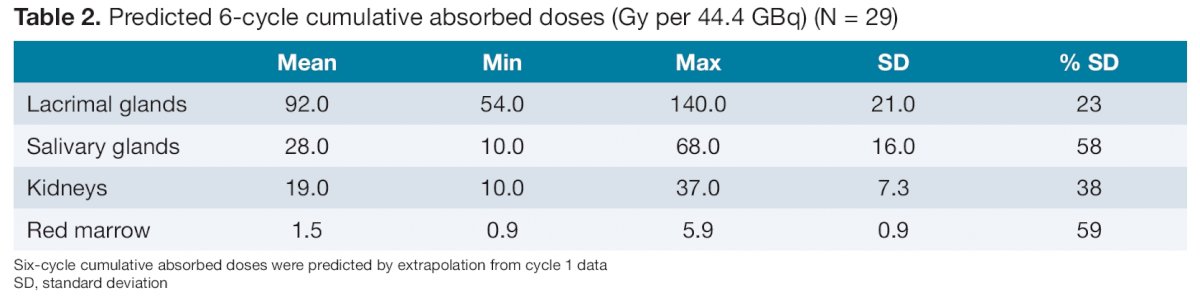
Within the context of greater VISION trial, the authors performed dosimetry in a separate cohort of 29 non-randomized participants at four German sites. These patients received 177Lu‑PSMA‑617 (7.4 GBq per cycle) plus SOC every 6 weeks for a maximum of 6 cycles. All patients included in this dosimetry sub-study underwent planar whole-body scintigraphy scans and single-photon emission computed tomography/computed tomography (SPECT/CT) scans of the upper and lower abdomen at 2-, 24-, 48-, and 168-hours after the first administration of 177Lu-PSMA-617. Additionally, blood and urine samples were collected throughout the first treatment cycle. 177Lu-PSMA-617 dosimetry outcomes were based on biodistribution, assessed using whole-body conjugate planar-image data, SPECT/CT image data, blood assay data and urinary excretion data. Organ Level INternal Dose Assessment/EXponential Modeling (OLINDA/EXM) software code version 2.2 was used for radiation exposure estimation and results were expressed as absorbed dose per unit activity (Gy/GBq). Further, the cumulative estimated absorbed dose (Gy) over the entire 6 cycle treatment course (44.4 GBq cumulative activity) was extrapolated from cycle 1 data.
The authors found that radiation-absorbed doses per unit activity were highest in the lacrimal glands (mean 2.1 Gy/GBq (standard deviation [SD], 0.47)) and the salivary glands (mean 0.63 Gy/GBq (0.36)). In contrast, the kidneys received 0.43 Gy/GBq (SD, 0.16) and the blood-based red marrow dose was 0.035 Gy/GBq (0.02).

Extrapolated to a full 6-cycles, the cumulative estimated absorbed dose was 92 Gy (SD, 21) in the lacrimal glands, 28 Gy (16) in the salivary glands, 19 Gy (7.3) in the kidneys and 1.5 Gy (0.90) in the red marrow.
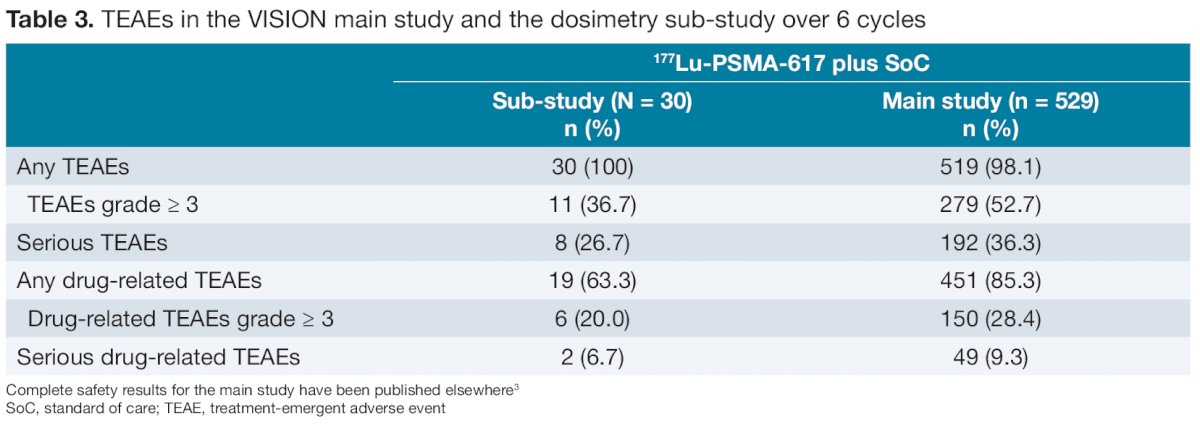
During cycle 1, 20% of patients had at least one hematological AE of Common Terminology Criteria for Adverse Events (CTCAE) grade ≥ 2 event though no patient experienced a grade 2 or greater renal or lacrimal gland toxicity, and only two patients had a grade 1 salivary gland AE.
Presented by: Ken Herrmann, MD, MBA, Professor and Chair of the Department of Nuclear Medicine, Universitatsklinikum Essen, Essen, Germany


