(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Arash Rezazadeh discussing dosing, safety, and pharmacokinetics of combination therapy with darolutamide, ADT, and docetaxel in patients with metastatic hormone-sensitive prostate cancer (mHSPC) in the ARASENS study.
In ARASENS (NCT02799602), darolutamide in combination with ADT and docetaxel significantly reduced the risk of death by 32.5% (HR 0.68; 95% CI 0.57–0.80) vs placebo + ADT + docetaxel in patients with mHSPC.1 Furthermore, incidences of treatment-emergent adverse events were similar between treatment groups. At the 2023 GU ASCO annual meeting, Dr. Rezazadeh and colleagues reported the dosing, safety, and pharmacokinetics of coadministration of darolutamide and docetaxel with ADT.
Patients with mHSPC were randomized 1:1 to darolutamide 600 mg twice daily or placebo, plus ADT and docetaxel (75 mg/m2 q21d for 6 cycles). The trial design for ARASENS is as follows:
The effect of darolutamide on docetaxel pharmacokinetics was assessed by noncompartmental analysis from the first 25 patients with dense pharmacokinetics data and by population pharmacokinetics for all patients. Darolutamide pharmacokinetics from ARASENS were compared with pharmacokinetics data from ARAMIS (2 NCT02200614; without docetaxel) to evaluate the impact of docetaxel on darolutamide pharmacokinetics.
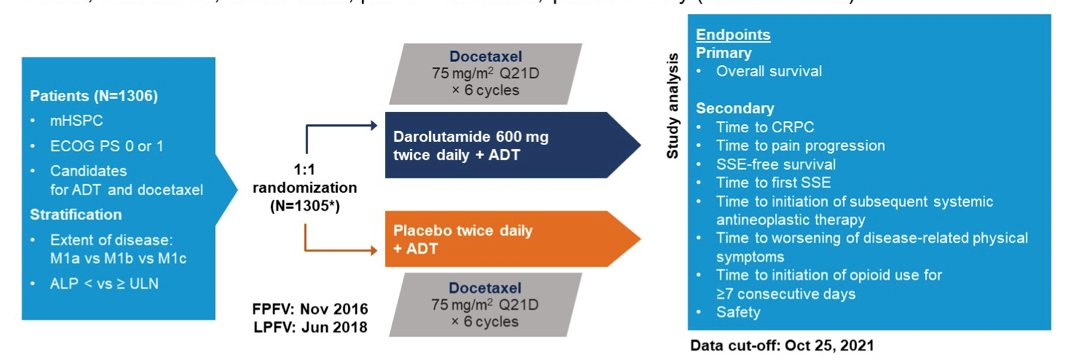
Of 1,306 randomized patients, 1,305 were included in the full analysis set (darolutamide, n=651; placebo, n=654). The median treatment duration was longer with darolutamide vs placebo (41.0 vs 16.7 months):
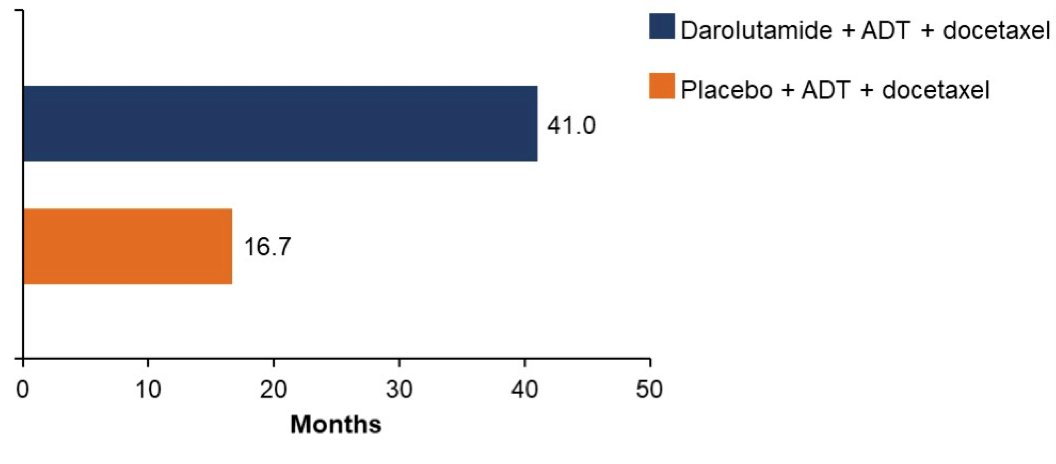
and more darolutamide-treated patients (45.9% vs 19.1%) were still receiving treatment at primary analysis cutoff (October 25, 2021):
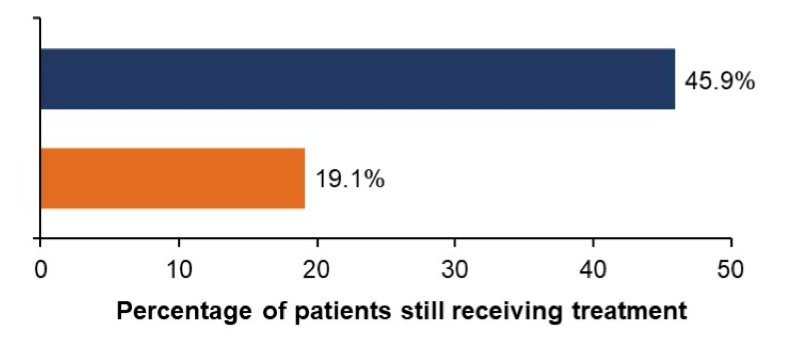
Almost all patients completed 6 cycles of docetaxel in both groups (darolutamide, 87.6% vs placebo, 85.5%). The proportion of patients requiring docetaxel dose modification (interrupted/delayed or reduced) was similar between groups (darolutamide, 60.0% vs placebo, 62.9%). Treatment-emergent adverse events led to discontinuation/reduction of docetaxel in 8.0%/19.9% of darolutamide patients and 10.3%/19.5% of placebo patients:
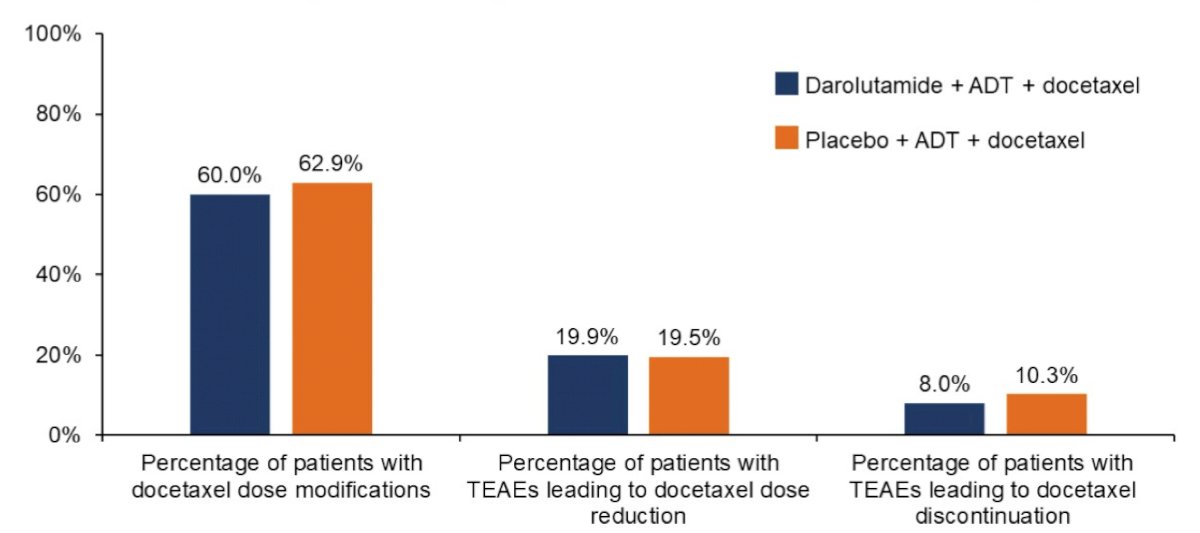
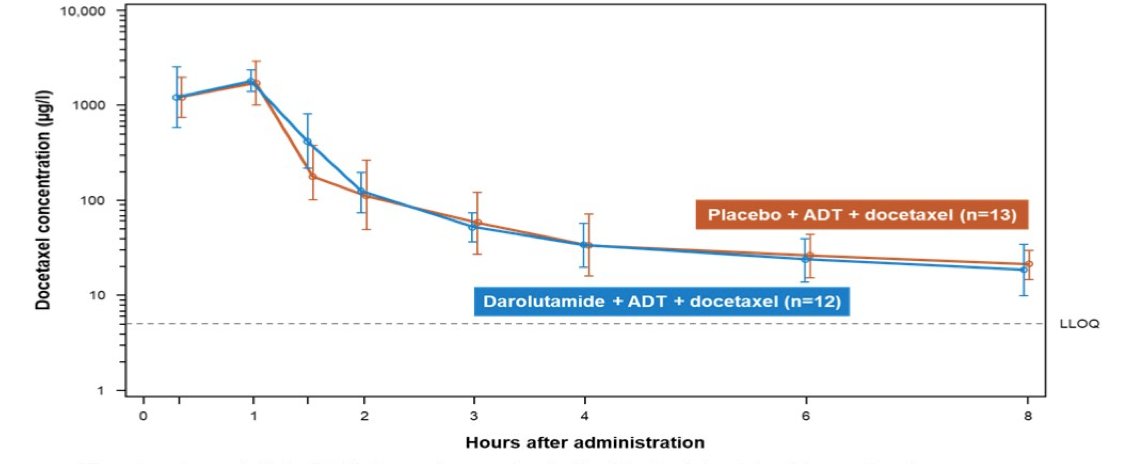
Population pharmacokinetics analysis indicated that docetaxel pharmacokinetics in ARASENS was generally consistent with that in the literature. A slight numeric increase in docetaxel exposure was observed in the darolutamide + docetaxel + ADT arm, with 15% higher maximum plasma concentration (geometric mean, 1.93 vs 1.68 µg/mL) and 6% higher area under the concentration-time curve (AUC0-tlast within an 8-hour sampling interval, 2.10 vs 1.99 µg.h/mL) vs placebo + docetaxel + ADT. This small numeric increase is likely not clinically relevant given the variability in docetaxel exposure (coefficient of variation, 23%–54%). Pharmacokinetics meta-analysis of ARASENS and, which considered patients’ intrinsic characteristics as covariates (eg. age, body weight, region), indicated a 10% lower AUC0-12ss of darolutamide in patients receiving docetaxel vs those not receiving docetaxel, which is not considered clinically relevant. Docetaxel plasma concentrations with and without darolutamide were overlapping at most time points, indicating no effect of darolutamide on docetaxel pharmacokinetics:
Dr. Rezazadeh concluded his presentation discussing dosing, safety, and pharmacokinetics of combination therapy with darolutamide, ADT, and docetaxel in patients with mHSPC in the ARASENS study with the following concluding messages:
- The combination of darolutamide + docetaxel + ADT increases overall survival with similar overall incidence of treatment-emergent adverse events and no observed drug-drug interactions between darolutamide and docetaxel
- Darolutamide can be effectively and safely administered with docetaxel in patients with mHSPC without clinically relevant changes in pharmacokinetics of darolutamide or docetaxel
Presented by: Arash Rezazadeh, MD, the University of California Irvine Medical Center, Orange, CA
Co-Authors: Bertrand F. Tombal, Maha H. A. Hussain, Fred Saad, Karim Fizazi, Cora N. Sternberg, E. David Crawford, Shivani Kapur, Weijiang Zhang, Bart Ploeger, Rui Li, Iris Kuss, Carsten Zieschang, Sabine Wittemer-Rump, Matthew Raymond Smith
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246.


