San Francisco, California (UroToday.com) Metastatic castration-resistant prostate cancer (mCRPC) is a molecularly heterogeneous disease, with between 20 and 25% of patients with mCRPC harboring deleterious alterations in DNA damage repair genes, including those with direct or indirect roles in homologous recombination repair (HRR). These gene alterations, of which BRCA1, BRCA2, and ATM are the most well-characterized, can confer sensitivity to PARP inhibition. The PROfound study showed that olaparib provides a statistically significant improvement in radiographic progression-free survival versus physician’s choice of enzalutamide or abiraterone in mCRPC patients with alterations in genes with a direct or indirect role in the HRR pathway.1 It is the largest study to date to prospectively screen patients with a qualifying alteration of >=1 of 15 prespecified genes by central tumor tissue next-generation sequence testing. At the Prostate Cancer Session at the 2020 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU), Maha Hussain reported additional findings of prospective central tumor testing of samples found in the PROfound study.
An investigational clinical trial assay based on the FoundationOne CDx™ NGS test developed in partnership with Foundation Medicine®, Inc, was used to prospectively identify patients with qualifying alterations in ≥1 of 15 prespecified genes, including BRCA1, BRCA2 or ATM. For the collection of new samples, sites were instructed to use standard formalin fixation methods in order to preserve nucleic acid integrity. Tumor testing was conducted centrally using archival or recent biopsy from primary or metastatic tissue.
Among 4,047 patients who submitted tumor samples, 2,792 (69%) were successfully sequenced and yielded a biomarker status. Categories for test failure (n=1255; 31%) were pathology review failure in 277 (6.8%) patients (eg estimated tumor fraction <20% or tumor volume <0.2 mm2), DNA extraction failure in 533 (13.2%) patients, and failure after DNA extraction in 280 (6.9%) patients, with 165 (4.1%) pts in >1 category. Regarding the sample disposition, approximately two-thirds of samples were from core needle biopsies, although higher success rates were observed with larger samples (ie prostatectomy). Samples were mainly from the prostate gland, with <5% from bone. Most samples were from archived tissue of primary tumors, whereas only ~10% were from newly collected tissue. As follows are the tissue testing success rates for archived versus newly collected samples and primary versus metastatic tumor samples: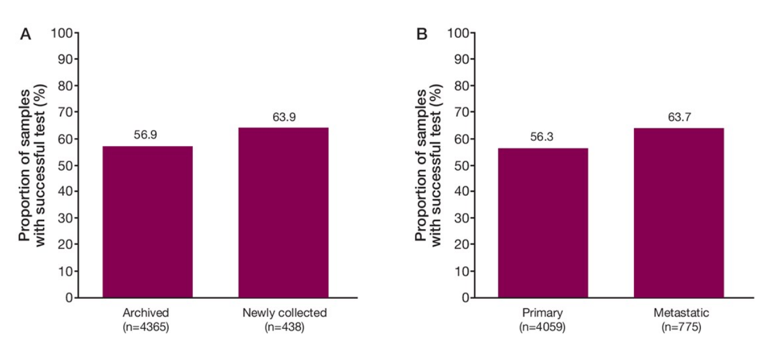
The age of samples assessed ranged from <1 year to >10 years. As expected, higher success rates were seen in more recently collected samples than older samples. This may be due to nucleic acid degradation or older samples depending on storage conditions suggesting that newer samples yield better results. However, almost 50% of samples aged >10 years were still able to yield genomic results. 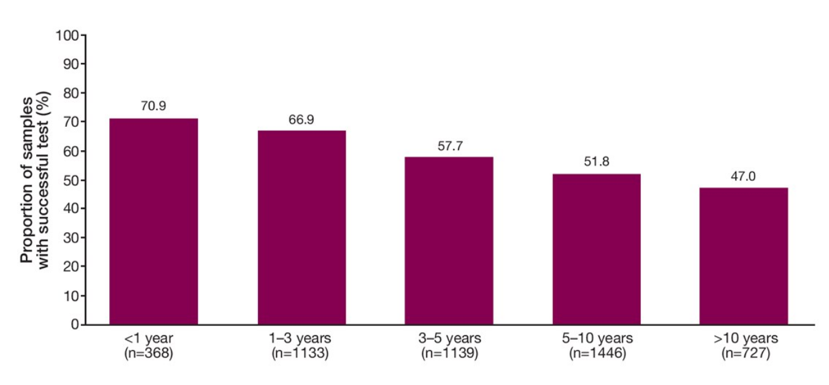
The PROfound study has demonstrated that tissue testing to identify HRR alterations in men with mCRPC is feasible. The majority of samples were from archival diagnostic biopsy or resection tissue. Although the testing success rate was higher with more recently collected samples, a successful test was possible with both archival and newly collected samples. Careful selection of high-quality tumor tissue samples is key to ensure success both at pathology review and during downstream steps of the NGS tissue testing process.
Clinical trial information: NCT02987543
Presented by: Maha Hussain, MD, Professor of Medicine, Deputy Director of the Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Northwestern University, Chicago, Illinois
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
Reference:
1. Hussain, M., J. Mateo, K. Fizazi, F. Saad, N. D. Shore, S. Sandhu, K. N. Chi et al. "PROfound: Phase III study of olaparib versus enzalutamide or abiraterone for metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR) gene alterations." Annals of Oncology 30 (2019): v881-v882.


