(UroToday.com) At the 2022 American Society of Clinical Oncology Annual Meeting held in Chicago and virtually, the poster session focused on Prostate, Testicular, and Penile cancers on Monday afternoon included a presentation from Dr. Alicia K. Morgans describing progression patterns of patients with non-metastatic castration resistant prostate cancer (nmCRPC) in the ARAMIS trial.
Darolutamide is a structurally distinct and highly potent androgen receptor inhibitor. Like apalutamide and enzalutamide, a randomized clinical trial of darolutamide (ARAMIS, NCT02200614) demonstrated significantly increased median metastasis-free survival by ̃2 years and improved overall survival in men with non-metastatic castration resistant prostate cancer (nmCRPC), with a favorable tolerability profile. The authors performed a post-hoc analysis of ARAMIS to evaluate the association between metastatic progression with prostate-specific antigen (PSA) and clinical progression and to describe the distribution of metastatic progression between groups.
As previously described, the ARAMIS trial enrolled patients with high-risk nmCRPC who were randomized in a 2:1 fashion to darolutamide (n=955) or placebo (n=554) while continuing androgen-deprivation therapy. The authors performed descriptive analyses using the primary data cutoff of September 3, 2018, utilizing the double-blind period of the ARAMIS trial. Following baseline, patients underwent conventional radiographic imaging every 16 weeks which was centrally reviewed. PSA and pain progression were defined per primary analysis.
In keeping with the overall results of the ARAMIS trial, metastatic progression was more common among patients randomized to placebo (28.5%) compared to darolutamide (13.6%). Among patients with evidence of metastatic progression, most patients had isolated progression in bone (darolutamide 46%, placebo 39%) or lymph node (darolutamide 32%, placebo 40%).

Those patients with radiographic progression had shorter median time from initial diagnosis to study treatment, compared to the overall ARAMIS population both among those treated with darolutamide (72.9 vs 86.2 months) and those randomized to placebo (74.4 vs 84.2 months). Of all patients with metastatic progression, baseline PSA levels (ng/mL) were similar between those randomized to darolutamide (12.6) and placebo (15.1).
Interestingly, prior to metastasis, patients on darolutamide had lower median PSA (16.7) compared to those receiving placebo (48.0). Further, patients randomized to darolutamide had median absolute/relative PSA decreases from baseline of -0.7/-3.2% as compared to an increase seen in patients receiving placebo of 29.5/181%.
Notably, PSA progression before metastasis was observed in only 55.6% (160/288) of patients, occurring in numerically fewer patients (45.4%) vs PBO pts (63.9%) (treatment difference 18.5%; nominal 95% CI 6.5%–30.6%).
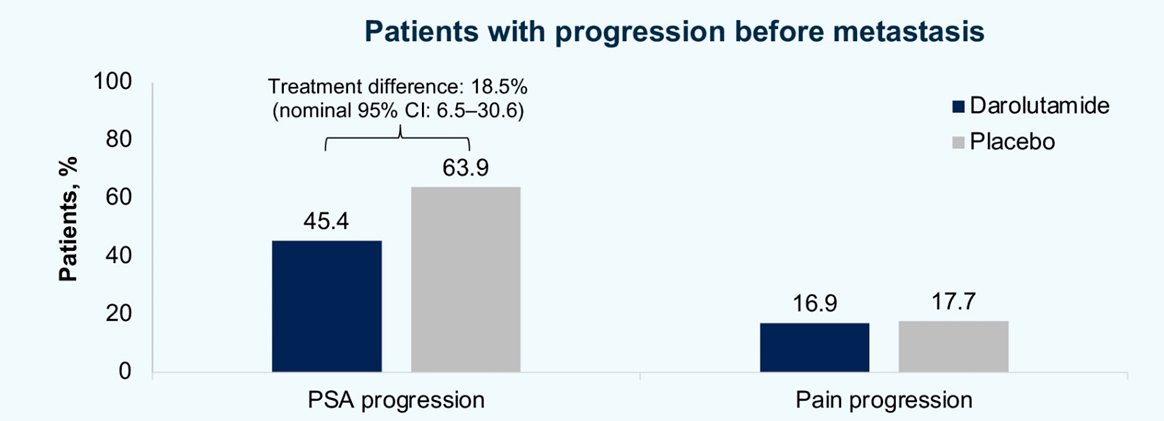
The median time between PSA progression and metastasis was 7.0 months among patients receiving darolutamide and 5.6 months among those receiving placebo. Pain progression before metastatic progression was rare and similar between groups (darolutamide 16.9%, placebo 17.7%).

The authors concluded that, while darolutamide significantly reduced risk of metastatic progression and improved overall survival in men with nmCRPC, it did not change the pattern of metastatic progression. Further, importantly, many patients with nmCRPC experienced metastatic progression without PSA progression, and pain progression was rare. These results support the use of imaging with PSA monitoring to properly identify disease progression in pts with nmCRPC.
Presented by: Alicia Morgans, MD, MPH Genitourinary Medical Oncologist, Medical Director of Survivorship Program at Dana-Farber Cancer Institute, Boston, Massachusetts.


