(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Poster Session A focussed on the care of patients with prostate cancer. Dr. Fizazi presented a poster focused on the effects of comorbidities and concomitant medications among patients on the ARAMIS trial which evaluated darolutamide in non-metastatic castration-resistant prostate cancer (nmCRPC). Most patients with nmCRPC are older, have comorbidities, and take concomitant medications. Thus, this is a clinically relevant question and may be particularly notable in the prostate cancer treatment space given that darolutamide has low potential for drug−drug interactions.
While previously presented and published, to summarize, the ARAMIS trial enrolled men with nmCRPC and randomized them in a 2:1 fashion to receive darolutamide (n=955) or placebo (n=554) while continuing androgen-deprivation therapy. These subgroup analyses utilized data as of the final data cut-off of November 15, 2019 and patients were stratified based on the number of comorbidities (≤ and >6) and concomitant medications (≤ and >10) in the double-blind period. HRs (95% CIs) were determined from univariate analysis using Cox regression.
The majority of patients had ≥6 comorbidities (53%; 795/1509) or received ≥10 concomitant medications (54%; 813/1509). The authors found consistent evidence of a beneficial protective effect of darolutamide on overall survival, across all subgroups.
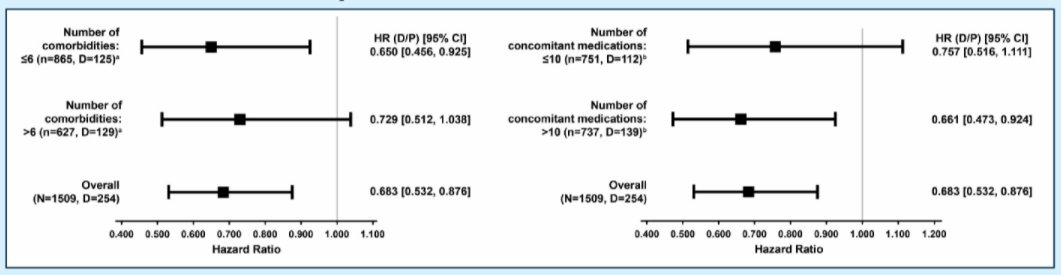
Additionally, when the authors considered subgroup analyses according to comorbidity groups, they found a consistent effect for metabolic, cardiovascular, and other comorbid disorders. Further, there was a consistent benefit across subgroups of patients receiving concomitant medications for gastrointestinal/metabolic disorders, CV disease, urologic disorders, and pain/inflammation.
When the authors further assessed toxicity, the incidence of AEs and AEs leading to treatment discontinuation with darolutamide was comparable to placebo across subgroups by the number of comorbidities and concomitant medications.
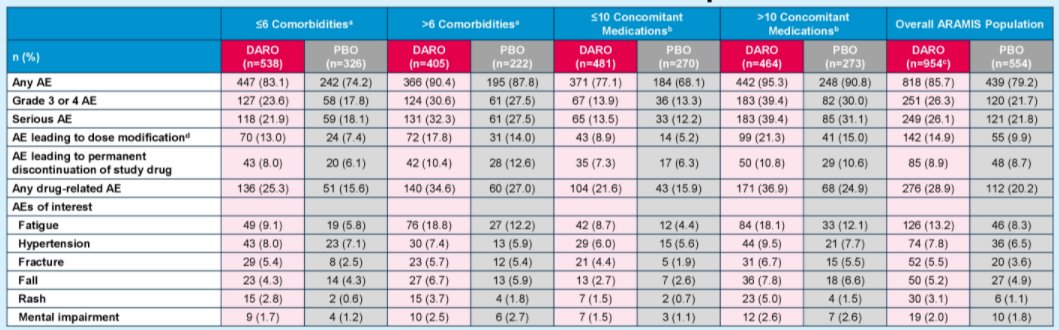
Thus, the authors conclude that both the safety and the efficacy of darolutamide in nmCRPC is consistent across subgroups of patients defined based on comorbidity and concomitant use of medication.
Presented by: Karim Fizazi PhD, MD, Gustave Roussy and University of Paris-Saclay, Villejuif, France

