We aimed to utilize the National Surgical Quality Improvement Program (NSQIP) database in conjunction with an institutional database to determine DVT/PE rates following RPLND and to determine the rate and impact of post-operative prophylactic anticoagulation (AC) for the past 10 years.
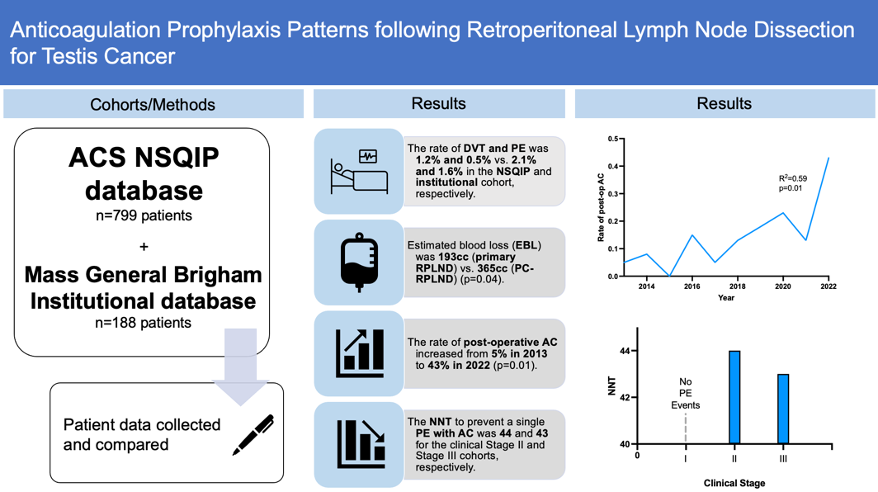
We found that the rate of DVT and PE following RPLND for testis cancer was 1.2% and 0.5% versus 2.1% and 1.6% in the NSQIP and institutional cohort, respectively. Using data from our institutional cohort, we found that the rate of post-operative AC increased from 5% in 2013 to 43% in 2022. Patients from the institutional database were stratified into cohorts by clinical stage at the time of RPLND. No Stage I patients developed PEs and no Stage I patients were prescribed AC. The number needed to treat (NNT) with AC prophylaxis to prevent a single PE was found to be 44 and 43 for the Stage II and Stage III cohorts, respectively.
In summary, we found the rate of DVT following RPLND to be relatively low. Nonetheless, the rate of AC prophylaxis following RPLND has increased dramatically in the past decade with no related adverse events. Using the NNT to prevent PE with AC, we calculated the benefit-cost ratio (BCR); the BCR of one month of treatment is 0.41 (indicating a net negative value) whereas the BCR for one week of treatment is 1.64 (indicating a net positive value). As such, we concluded that AC following RPLND was safe and efficacious (especially in higher stage disease). The optimal duration of AC therapy must be clarified before recommendations can be made with respect to its cost-benefit efficacy, and prospective clinical trials may be beneficial with this analysis.
Written by: Vincent D. D’Andrea1 & Timothy N. Clinton2
- Department of Surgery, Division of Urology, Harvard Medical School, Brigham & Women's Hospital, Boston, MA
- Department of Surgery, Division of Urology, Harvard Medical School, Brigham & Women's Hospital, Boston, MA; Harvard Medical School, Dana-Farber Cancer Institute, Boston, MA.


