We identified that cabozantinib is frequently used in the immuno-oncology refractory treatment landscape, but real world data examining the activity of the drug is missing.1 Furthermore, 2L regulatory indications for cabozantinib worldwide are primarily limited to patients who had received prior VEGFi treatment, to reflect the inclusion criteria of METEOR.2 In many jurisdictions, this restricts therapeutic use of cabozantinib to third line (3L) for patients who are treated with 1L IPI-NIVO and 2L VEGFi. Integration of cabozantinib into a shifting therapeutic paradigm requires real world evidence given the absence of randomised prospective data.
Given this, we used the IMDC database to identify clinically useful endpoints [ the objective response rate, time to treatment failure (TTF), and overall survival (OS)] of 2L cabozantinib after ipilimumab/nivolumab, immuno-oncology VEGF receptor inhibitor combinations, pazopanib or sunitinib or other 1L therapies. No formal calculations of sample size or statistical power were performed given the observational nature of this cohort analysis. Data analysis was descriptive in nature and any conclusions should be couched in that context.
We identified Three hundred and forty-six patients that received 2L cabozantinib (13 missing due to incomplete survival data). The Sankey diagram that outlines their treatment sequencing is outlined below
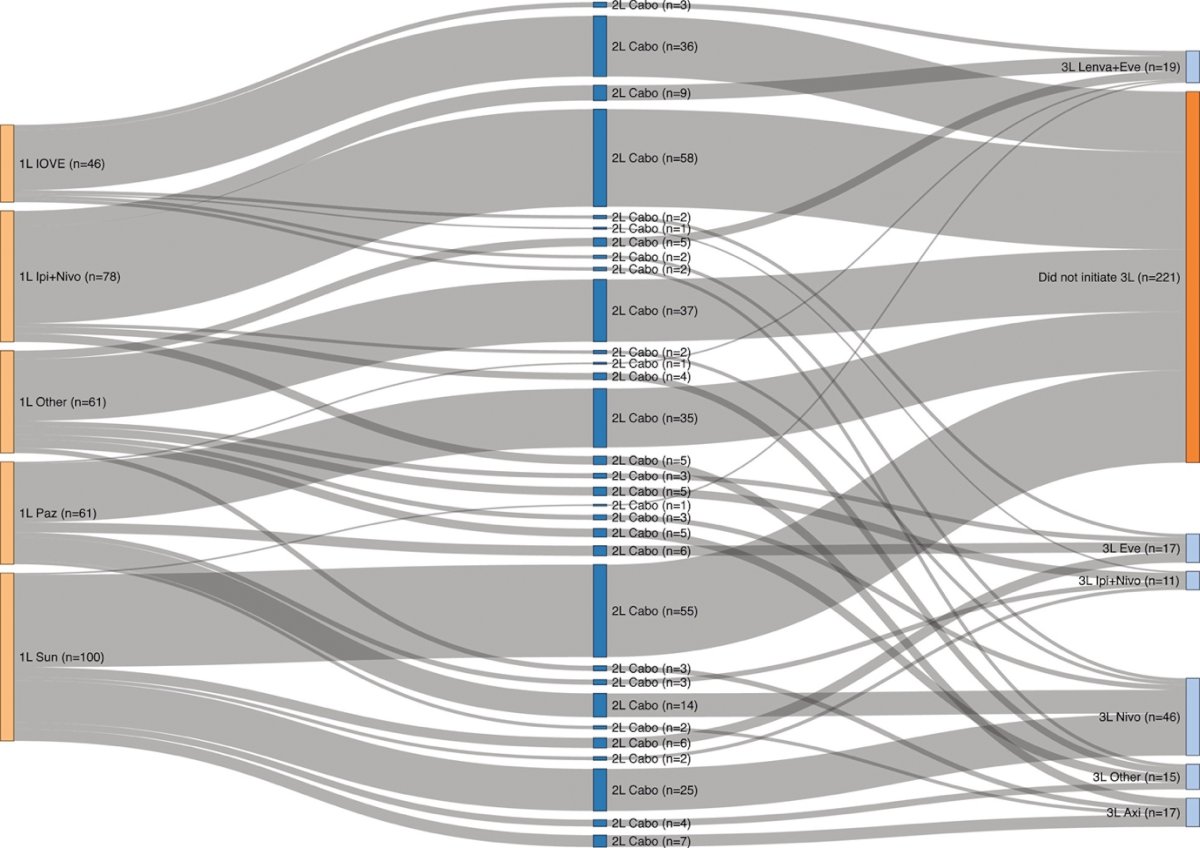
Comparing baseline characteristics of patients that received 2L cabozantinib, by 1L therapy, there were no statistically significant differences based on variables such as age at initiation of cabozantinib, gender, sites of metastases, or sarcomatoid differentiation. Imbalances in IMDC risk were noted, as expected based on regulatory approvals, with significantly more IMDC poor risk patients treated with 1L IPI-NIVO (37.5% vs. 17.1% IOVE vs. 22.9% 1L PAZ/SUN P= .028).
In our multivariable IMDC risk group adjusted regression analysis, we could not detect a significant difference in OS in 2L cabozantinib treated patients based on their type of 1L therapy. No difference was found when 1L IPI-NIVO (reference group) was compared with 1L IOVE: a nonsignificant HR of 1.73 (0.83 - 3.62 P = .14). In a further sensitivity analysis, when other common 1L approaches such as 1L PAZ/SUN were compared to 1L IPI-NIVO, again no statistically significant difference in OS was identified.
Given these findings, with similar time to event data and overlapping time to treatment failure and overall survival curves, alongside the absence of a difference detected on adjusted cox regression analysis, we concluded that there is comparable activity of 2L cabozantinib in our dataset, independent of preceding 1L therapy. Given the regulatory and reimbursement landscape globally often limits the use of cabozantinib to patients post VEGF inhibitor monotherapy, this may inform the inclusion of cabozantinib in the treatment sequence after now contemporary standard of care 1L IO3 combination approaches.
Limitations of our work include large confidence intervals in our regression analysis, suggesting a potential lack of statistical power to make such descriptive comparisons. Furthermore, an inability to identify statistical differences between outcomes based on varying preceding lines of treatment does not necessarily imply equivalence, given the nature of this observational data. Mature follow up and future accrual will help clarify any interaction for activity of 2L cabozantinib in terms of OS by type of 1L therapy received.
This data adds to the body of work supporting the role of VEGFi TKI in later lines of therapy and provides outcome benchmarks for 2L cabozantinib in a contemporary setting. These are real world benchmarks with which to counsel our patients.
Written by: Vishal Navani, J Connor Wells, Devon J Boyne, Winson Y Cheung, Darren M Brenner, Bradley A McGregor, Chris Labaki, Andrew L Schmidt, Rana R McKay, Luis Meza, Sumanta K Pal, Frede Donskov, Benoit Beuselinck, Maxwell Otiato, Lisa Ludwig, Thomas Powles, Bernadett E Szabados, Toni K Choueiri, Daniel Y C Heng
Tom Baker Cancer Centre, Calgary, Canada. Electronic address: ., BC Cancer Agency, Vancouver, Canada., University of Calgary, Calgary, Canada., Tom Baker Cancer Centre, Calgary, Canada; University of Calgary, Calgary, Canada., Dana Farber Cancer Institute, Boston, USA., University of California San Diego, Moores Cancer Center, La Jolla, United States., City of Hope Comprehensive Cancer Center, Duarte, United States., University Hospital of Southern Denmark, Esbjerg, Denmark., University Hospitals Leuven, Leuven, Belgium., University of Michigan, Ann Arbor, USA., Ipsen Biopharmaceuticals, Canada., Barts Cancer Institute, Queen Mary University of London, London, United Kingdom., Tom Baker Cancer Centre, Calgary, Canada.Disclosure: This study was funded by Ipsen, who provided input into the design of the study. Ipsen played no role in the collection and analysis of the data or drafting the manuscript. The funder provided input into the manuscript for scientific accuracy.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- Gan CL., Dudani S., Wells JC. et al. "Cabozantinib real-world effectiveness in the first-through fourth-line settings for the treatment of metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium." Cancer Med. 2021; 10: 1212-1221
- Choueiri TK., Escudier B., Powles T. et al. "Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial." Lancet Oncol. 2016; 17: 917-927
- Navani V., Heng D. "Treatment Selection in First-line Metastatic Renal Cell Carcinoma—The Contemporary Treatment Paradigm in the Age of Combination Therapy."JAMA Oncol. 2022. 8(2):292-299.
Read the Abstract


