Published Date: June 2016
Prostate cancer [PCa] affects 1 man in 7 in the United States, making this the most commonly diagnosed non-cutaneous cancer in males. Although an ever-increasing number of treatment options exist, an estimated 26,100 men will still die of the disease in the US in 2016, generally after primary local and systemic treatments for prostate cancer have failed.
Prostate cancer [PCa] affects 1 man in 7 in the United States, making this the most commonly diagnosed non-cutaneous cancer in males. Although an ever-increasing number of treatment options exist, an estimated 26,100 men will still die of the disease in the US in 2016, generally after primary local and systemic treatments for prostate cancer have failed.
One factor contributing to this statistic is the frequent inability of current diagnostic methods to reliably detect the exact location(s) of disease relapse at a time when curative treatment is still possible.
The dilemma of biochemical recurrence (BCR): Up to a third of men treated for prostate cancer will experience recurrent disease, , most often detected only by rising Prostate Specific Antigen [PSA] levels. Conventional imaging tools such as computerized tomography [CT] and bone scintigraphy [BS] frequently fail to identify the site of recurrent disease, presenting a serious challenge to urologists and radiation oncologists charged with the selection of secondary treatment, and causing significant anxiety for these patients.
While PCa recurrence may occur locally in the prostate gland or prostate bed, and/or in local lymph nodes in the pelvis, recognition of distant lymph node, bone or other tissue involvement requires different treatment choices. Potentially curative techniques such as salvage lymphadenectomy, radiotherapy or cryotherapy may be used for local recurrences, especially at lower PSA levels, whereas systemic approaches such as the use of anti-
hormonal therapy and/or chemo or immunotherapy may be recommended in the presence of distal metastatic disease.
Conventional imaging: Conventional diagnostic imaging with CT and BS is typically of limited utility until PSA values rise to 10-20 ng/ml. Kane et al reported that patients with BCR after radical prostatectomy have a low probability of a positive BS (9.4%) or a positive CT scan (14.0%) within 3 years of biochemical recurrence. Similarly, in men with BCR at a median time of 5 years post-surgery, of the 380 bone scans done among hormone-naive subjects, only 24 (6%) were positive, with 356 (94%) negative for metastasis. CT images, although highly specific when positive, do not generally raise suspicion if the involved lymph node is <1 cm in diameter, even if the node is cancerous. 18F FDG PET is of limited use in BCR because uptake in PCa is generally suboptimal until the patient has metastatic castrate resistant disease, and physiologic excretion of FDG in the bladder may interfere with image interpretation of adjacent structures in the pelvis. However, salvage radiation therapy (SRT) to the prostate should be initiated well before a PSA of 10 – 20 ng/mL is reached, as studies have demonstrated that outcomes are better if SRT is initiated at low (e.g. <0.5 ng/mL) PSA values and refs therein and is not likely to be curative if the disease has progressed to the point where it can be seen on a bone scan.
Enter Axumin™: On May 27, 2016 the FDA approved Axumin [fluciclovine F 18 Injection; Blue Earth Diagnostics Ltd, Oxford UK] for PET imaging in men with suspected prostate cancer recurrence based on elevated PSA levels following prior treatment. The approval of Axumin ushers in a new era of F18 PET/CT for the detection of recurrent prostate cancer in the USA. Data submitted to FDA included results from prospective studies at Emory University and the University of Bologna and from clinical use at two sites in Norway; the pooled data for n= 595 subjects were retrospectively analyzed. Overall, fluciclovine F 18 PET/CT detected sites of recurrence in 68% (403/595) of patients. For patients with baseline PSA values in the lowest quartile (<0.79 ng/mL, n=128), 75 patients (59%) were negative with fluciclovine F 18 PET/CT, but 53 (41% of patients) had positive fluciclovine scans; 13 (10%) had prostate bed findings only, and 40 (31%) had extra-prostatic disease. As the location of disease recurrence affects appropriate therapy selection, this finding is highly relevant.
How it works: Fluciclovine is a synthetic amino acid that is preferably taken up by amino acid transporters in tissues. These transporters, specifically ASCT2 and LAT1, bring amino acids such as glutamine and leucine into cells, where they are used for protein synthesis, cell growth and metabolism. Cancer cells often have an increased requirement for amino acids to support increased metabolism and proliferation. Imaging studies with histological confirmation have demonstrated that uptake of fluciclovine F 18 is enhanced in PCa in the prostate bed, involved lymph nodes and bony metastases. Thus, this biochemically relevant localization method may be useful to evaluate suspected nodal or metastatic disease where confirmation or exclusion of pelvic and/or distant disease would directly influence patient management.
Fluciclovine image: As an example, the image to the right is a case from a 61 year-old male with PSA rising to 0.4 ng/mL after robotic-assisted laparoscopic prostatectomy. Fluciclovine F 18 PET/CT detected an 8 mm lymph node proximal to the rectal wall, rendering delivery of salvage radiotherapy problematic. The patient went on to receive hormonal therapy. (See Figure 1)

Comparison to other imaging tools: Studies comparing the agent to the gamma-emitting agent ProstaScint, and to CT and 11C-choline have been reported, as have descriptions of its use in primary prostate cancer and in therapy planning. In an NIH funded prospective RO1 study at Emory University, 115 patients with BCR and negative 99mTc bone scans after radical prostatectomy or radiotherapy were imaged with fluciclovine F 18. The majority (n=93) also received 111In capromab pendetide (ProstaScint, a radio-labelled monoclonal antibody that binds to prostate-specific membrane antigen). This study compared the regional sensitivity and specificity of the two agents, relative to histology and clinical follow-up. Sufficient data for truth assessment (histology, response to therapy and clinical follow-up) were available for 91 patients with prostate/bed findings and for 70 patients with extra-prostatic involvement. The following results were found (See Figure 2)
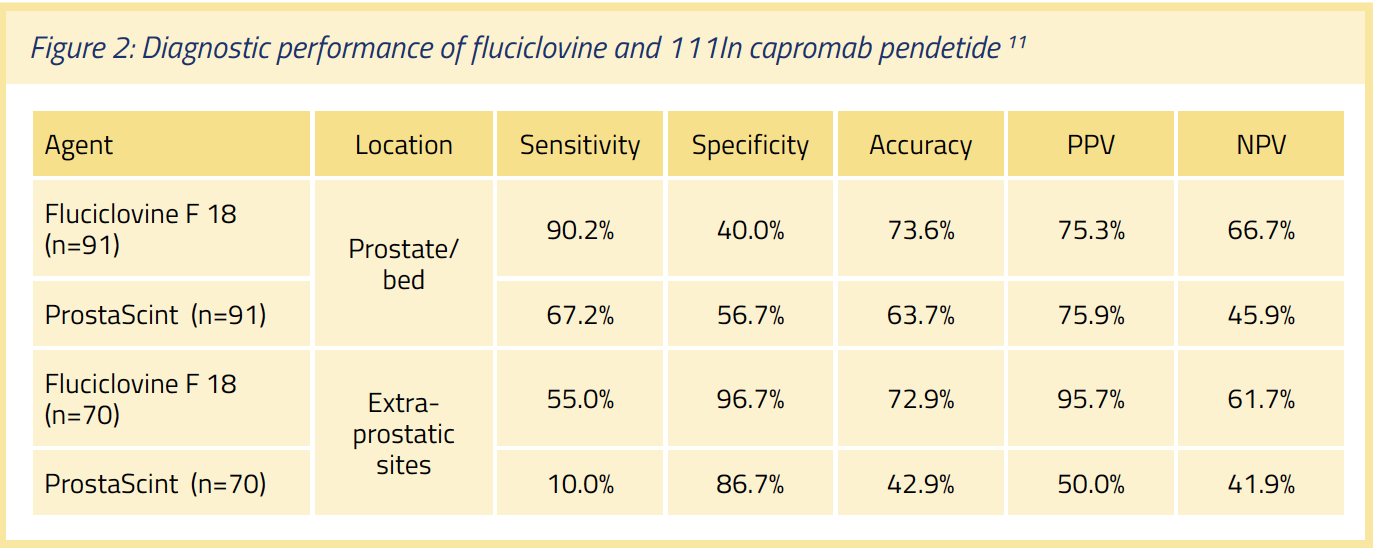
Of the 77 index lesions used to prove positivity, histological proof of PCa was obtained in 74 (96.1%). Fluciclovine F 18 identified 14 more positive prostate bed recurrences (55 vs 41) and 18 more subjects with extra-prostatic involvement (22 vs 4). The agent upstaged 25.7% of the patients. For fluciclovine, imaging was complete within 40 min post-injection (PI), in contrast to 111In capromab pendetide, where images were obtained at 3 days PI.
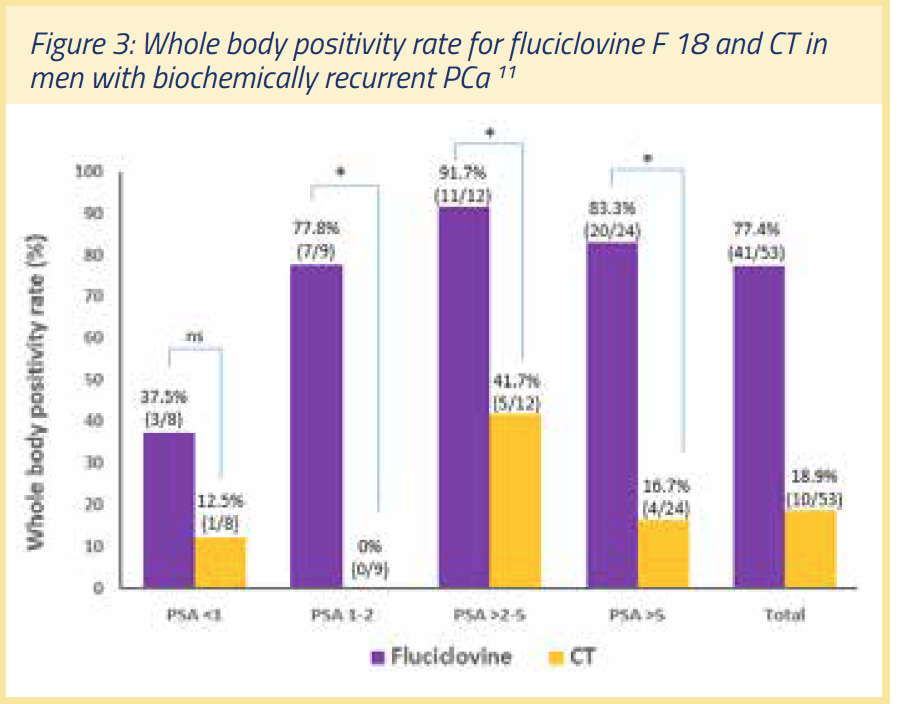
A secondary endpoint of the above study analyzed a subset (n=53) to compare the ability of CT and fluciclovine F 18 to detect recurrent disease (see Figure 3). On a whole-body basis, 41/53 fluciclovine PET/CT scans (77.4%) were positive, but only 10/53 scans (18.9%) were positive with CT. Of 33 patients with histological proof of disease, fluciclovine PET/CT detected disease in 31 (93.9%) but CT detected disease in only 4 (12.1%). The authors concluded that the diagnostic performance of fluciclovine PET/CT in recurrent prostate cancer is superior to that of CT, and provides better delineation of prostatic from extra-prostatic recurrence. Detection rate with fluciclovine F 18 was 37.5% at PSA values <1, and increased to 77.8% in a PSA range of 1-2 ng/mL.
Comparison to 11C-choline: A prospective study conducted at Bologna University enabled a within-subject comparison of the performance of fluciclovine F 18 PET and 11C-choline PET in patients with BCR post-radical prostatectomy (n=89). Follow-up at 1 year was used as the reference standard. Diagnostic performance was comparable for both agents at PSA values above 1 ng/mL, but fluciclovine F 18 imaging showed higher sensitivity in patients with low PSA levels (<1 ng/mL). 11C-choline has been approved for use in the detection of BCR at selected sites in the United States, but the 20 minute half-life of the 11C isotope limits its use to facilities with a nearby cyclotron. In contrast, the 110 minute half-life of F18 in fluciclovine improves the potential for broad patient access.
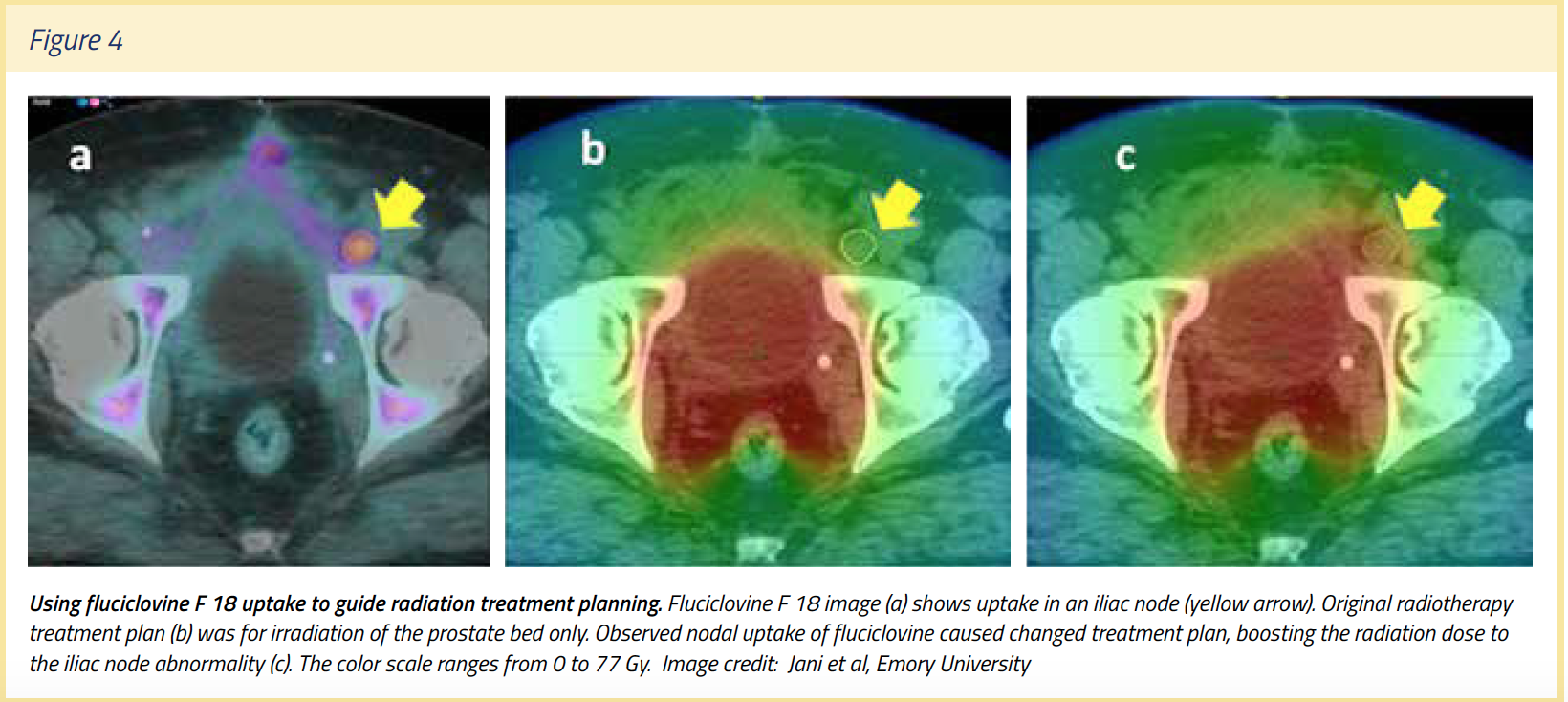
Radiation therapy planning: Schreibmann et al incorporated fluciclovine F 18 PET/CT into radiotherapy treatment planning to define prostate bed and lymph node target volumes in 41 patients. Inclusion of the fluciclovine F 18 images changed the planning volumes for 46 abnormalities (83%) of the total 55, with 28 (51%) located in the lymph nodes. Use of fluciclovine F 18 in post-prostatectomy radiotherapy planning was feasible and led to augmentation of the target volumes in the majority (30 of 41) of patients studied. (See Figure 4)
Important safety information: The recent FDA approval of fluciclovine F 18 was based on data from 877 subjects including 797 men diagnosed with PCa. Adverse reactions were reported in ≤1% of subjects during these clinical studies. The most common adverse reactions were injection site pain and/or redness, and dysgeusia (abnormal taste in the mouth). Although not yet observed, hypersensitivity reactions, including anaphylaxis, may occur in patients who receive radiopharmaceuticals, so emergency resuscitation equipment and personnel should be immediately available. Axumin use contributes to a patient's overall long-term cumulative radiation exposure, and safe handling practices should be used to minimize radiation exposure to the patient and health care providers.
As with any imaging agent, image interpretation errors can occur with fluciclovine PET imaging. A negative image does not rule out recurrent prostate cancer (if lesions are small, they may be below the resolution of the PET camera) and a positive image does not confirm its presence, as fluciclovine uptake may occur with other cancers and is also seen in tissue affected by benign prostatic hypertrophy (BPH) in primary prostate cancer. Clinical correlation, which may include histopathological evaluation, is recommended.
Coming soon to a radiopharmacy near you: FDA approval of Axumin is only the first step. The imaging agent is made on demand at specialized manufacturing facilities for the preparation of radioactive drugs and then shipped to imaging centers that have been trained to administer the product and interpret the images. The agent will become increasingly available in the coming months across the US through the national radiopharmacy network of Blue Earth Diagnostic’s U.S. commercial manufacturer and distributor, Siemens’ PETNET Solutions. For men with BCR, early detection of disease with Axumin (fluciclovine F18) and initiation of appropriate therapy may provide the potential for better long-term outcomes in prostate cancer.
Written by: Karen E. Linder, MS, PHD
References:
1. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics Accessed Aug 16 2016
2. J Mohler, RR Bahnson, B Boston, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw 2010;8:162-200.
3. JF Ward, ML Blute, J Slezak, et al. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol 2003;170:1872-76.
4. CJ Kane, CL Amling, PA Johnstone, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology 2003;61:607-11.
5. DM Moreira, MR Cooperberg, LE Howard, et al. Predicting bone scan positivity after biochemical recurrence following radical prostatectomy in both hormone-naive men and patients receiving androgen-deprivation therapy: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2014;17(1):91-96.
6. CY Yu, B Desai, L Ji, et al. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am J Nucl Med Mol Imaging 2014;4(6):580-601.
7. AJ Stephenson, PT Scardino, MW Kattan, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007;25(15):2035-41.
8. AxuminTM (Fluciclovine F 18 Injection) package insert. Blue Earth Diagnostics Ltd. August, 2016.
9.T Bach-Gansmo, C Nanni, PT Nieh et al. Multi-site experience of the safety, detection rate and diagnostic performance of fluciclovine (18F) PET/CT imaging in the staging of biochemically recurrent prostate cancer,” J Urology 2016; doi:10.1016/j.juro.2016.09.117
10. BC Fuchs, BP Bode. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol 2005;15(4):254-66.
11. DM Schuster, PT Nieh, AB Jani, et al. Anti-3-[18F]FACBC Positron Emission Tomography-Computerized Tomography and 111In-Capromab pendetide Single Photon Emission Computerized Tomography-Computerized Tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 2014;191(5):1446-53.
12. O A Odewole, FI Tade, PT Nieh, et al. Recurrent prostate cancer detection with anti-3-[F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 2016;43(10):1773-78.
13. C Nanni, L Zanoni, C Pultrone, et al. 18F-FACBC (anti 1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) versus 11C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging 2016; 43(9):1601-10.
14. E Schreibmann, DM Schuster, PJ Rossi, et al. Image-guided planning for prostate carcinomas with incorporation of anti-3-[18F]FACBC (Fluciclovine) positron emission tomography: workflow and initial findings from a randomized trial. Int J Radiation Oncol Biol Phys 2016;96(1):206-13.


