- Initial data from Clarity’s diagnostic Phase 1/2 trial, COBRA, confirms 64Cu-SAR-bisPSMA is safe and highly effective in detecting prostate cancer (PC) lesions in patients with biochemical recurrence (BCR).
- Confirmed unique benefit of 64Cu-SAR-bisPSMA for next-day imaging in this patient population, with more lesions and more patients with a positive scan identified via the next-day scan.
- In trial participants where standard of care (SOC) imaging was unable to detect any lesions, up to approximately 60% had lesions identified by same-day 64Cu-SAR-bisPSMA imaging and up to 80% on next-day imaging, with high specificity on both days.
- The number of lesions identified by 64Cu-SAR-bisPSMA on next-day imaging almost doubled compared to same-day imaging (increasing from up to 80 on Day 0 to up to 153 lesions on Day 1).
- Clinicians reported they would change their treatment plan in approximately 50% of patients due to 64Cu-SAR-bisPSMA scans, signaling a potential material improvement in patient care. Among those patients, two-thirds proceeded to receive focal and/or systemic therapy.
- Initial results from the COBRA trial will be presented at leading international oncology and nuclear medicine conferences.
- Phase 1/2 COBRA trial data to inform registrational Phase 3 trial in patients with BCR of PC.
Dr Neal Shore MD, FACS Lead Principal Investigator in the COBRA trial, Medical Director of Carolina Urologic Research Centre, commented, “We are very enthusiastic regarding the data from the early phase COBRA trial as 64Cu-SAR-bisPSMA was confirmed to be safe and effective in detecting PC lesions in patients with BCR who came into the study with negative or equivocal scans using SOC imaging. The trial demonstrates a clinical advantage of same-day and next-day imaging in detecting additional lesions as well as for potentially detecting low prostate-specific membrane antigen (PSMA) expressing or smaller lesions. When detecting recurrence of PC, it is important to visualise small lesions, as reliable imaging of low volume tumor burden can affect treatment decision making. In the COBRA trial, we saw that 64Cu-SAR-bisPSMA positron emission tomography (PET) informed potential changes in the treatment plan in approximately half of the patients. This makes a difference for patients who may then have insight into the location of their disease recurrence and thereby proceed to treatment of their cancer. We look forward to additional data readouts from the trial and presenting the results at future international medical conferences. If the data from the COBRA trial can be substantiated in a registrational Phase 3 study, and if approved by the United States Food and Drug Administration (US FDA), this PET PSMA modality will impact care for patients with PC.”
Clarity’s Executive Chairperson, Dr Alan Taylor, commented, “We are extremely excited with the initial findings from the COBRA trial as it further substantiates the many benefits of copper-64 (64Cu) and our optimised bisPSMA product in the diagnosis of PC. Combined with our clinical and pre-clinical trial data to date, this further validates SAR-bisPSMA as a potential best-in-class PSMA agent for the diagnosis (with 64Cu) and subsequent treatment (with copper-67 [67Cu]) of PC. We have also seen the performance of 64Cu-SAR-bisPSMA in the real-world setting through our compassionate use program, which demonstrated the ability of 64Cu-SAR-bisPSMA to detect lesions in patients with BCR and a negative or equivocal SOC imaging (including 18F-DCFPyL and 68Ga-PSMA-11). These cases also showed a higher number of lesions and lesions with higher maximum standardised uptake values (SUVmax) on next-day imaging. Therefore, we were already aware of the benefits of later-timepoint imaging, but the high number of lesions detected on same-day imaging that were negative or equivocal on SOC imaging (e.g. bone scan, CT or PSMA PET), and the almost doubling of the number of lesions with next-day imaging, is remarkable. The COBRA study now validates previous observations in a larger group of patients under trial conditions.
“Most importantly, the high rate of detection of PC in up to 80% of patients that were negative or equivocal on SOC imaging further brings to light the low sensitivity issues of current SOC imaging. We adhere to the highest standards and methods of clinical development as we pride ourselves on high quality science at Clarity. As such, the COBRA trial design was built to reflect the gold standard in clinical research and took a very conservative approach. Given we identified a large number of lesions with 64Cu-SAR-bisPSMA that were not visible with SOC scans, a number that was not anticipated when the protocol was prepared, it was not feasible nor ethical to verify all of these lesions with biopsy when caring for the patient. Therefore, our investigators were not able to confirm the true positivity of a very large number of lesions. Furthermore, among the patients that were excluded from the assessment of the efficacy endpoints because they had initiated systemic therapy while on study (which is not allowed according to the trial protocol), the majority had positive 64Cu-SAR-bisPSMA scans. As this was a first-in-human study in BCR PC patients, we have learnt significantly more about our product relative to current SOC imaging and will consider this potentially much higher level of detection and sensitivity of 64Cu-SAR-bisPSMA when structuring the Phase 3 trial design in this patient group.
“As can be seen from this study, and the clinicians reporting that they would change their treatment plan in response to this data, the increase in sensitivity with bisPSMA is incredibly important when assessing the return of PC in BCR patients. It is equally important for patients at initial staging of their disease, when they are considering their first course of definitive therapy, in order to understand whether their PC has metastasised or not from the primary lesion prior to prostatectomy. This is an immediate unmet medical need, which we are addressing with our Phase 3 CLARIFY trial2. Current SOC imaging fails to do this for a large percentage of men who eventually experience BCR of their PC. As such, there is an immediate opportunity for bisPSMA to address the entire market of PSMA imaging as we move closer to our ultimate goal of improving treatment outcomes of people with cancer.
“In addition to the clinical benefits, 64Cu-SAR-bisPSMA has a number of logistical and manufacturing advantages in comparison to other currently used PSMA agents. The ready-to-use product has been shipped to the trial sites in the US from a central manufacturing facility on-demand and on time, providing flexibility and reliability to the patients and their treating staff. This facilitates expanding the radiopharmaceutical field into the large oncology market, minimising logistical hindrances associated with the current generation of radio-diagnostics, and helping to focus on the needs of patients and their clinicians. Furthermore, the longer shelf-life of 64Cu-based products, coupled with the advantages of the SAR Technology, could offer access to PSMA PET for PC patients in underserved and broad geographical areas, which is one of the limitations of the approved PSMA agents due to their short half-life.”COBRA trial design & quality
The US-based COBRA study (Copper-64 SAR-bisPSMA in Biochemically Recurrent prostAte cancer) was a first-in-human trial of 64Cu-SAR-bisPSMA in patients with BCR of PC. It was a multi-centre, single-arm, non-randomised, Phase 1/2 diagnostic imaging study of 64Cu-labelled SAR-bisPSMA (64Cu-SAR-bisPSMA) administered to participants with BCR of PC following definitive therapy. The primary objectives of the trial were to investigate the safety and tolerability of 64Cu-SAR-bisPSMA as well as its ability to correctly detect recurrence of PC. Patients underwent PET/computed tomography (CT) scans on Day 0 and Day 1 (1-4h and 24±6h post-dose, respectively), which were interpreted by three blinded central readers. To determine the efficacy of 64Cu-SAR-bisPSMA imaging, the Day 0 and Day 1 PET/CT results of the central readers were assessed against a composite reference standard that was determined by an independent, blinded, central expert panel. The reference standard consisted of histopathology, follow-up SOC imaging and/or confirmed prostate specific antigen (PSA) response to focal therapy.
The design of the COBRA study followed advice from regulators to achieve the highest standards in clinical research in the BCR setting, in contrast to trials with other PSMA agents. Based on this guidance, the expert panel, who determined the reference standard, was blinded to the results of the 64Cu-SAR-bisPSMA scans and distinct from the central readers assessing the 64Cu-SAR-bisPSMA scans. This approach removed any potential biases in the assessment of the reference standard, which was not the case with previously approved PSMA tracers, where the reference standard was determined by readers having access to the PSMA scans investigated in the study, aiming to anatomically correlate the findings across the scans. Furthermore, a conservative approach was taken for the analysis of both co-primary endpoints. If a lesion identified on the 64Cu-SAR-bisPSMA scan was not biopsied and it was also not present on follow-up SOC imaging (a suboptimal reference standard with known low sensitivity), it was considered as false positive in the analysis by default. The higher standards in study design for COBRA compared to that used for other PSMA agents’ trials, along with various benefits intrinsic to 64Cu-SAR-bisPSMA not seen with currently approved products (e.g. higher uptake/retention and delayed imaging), make comparisons among other PSMA tracers and 64Cu-SAR-bisPSMA not viable. The methodology used in the COBRA trial underscores the ability of 64Cu-SAR-bisPSMA to correctly identify PC recurrence even when a higher level of rigour in study design is applied, making the transition of data to real world scenarios more probable.
Initial results from the COBRA trial
This study was the first-in-human trial of 64Cu-SAR-bisPSMA in patients with BCR of PC. Fifty-two patients with negative or equivocal SOC scans were enrolled and imaged, of whom 42 were included in the calculation of the efficacy endpoints. The median PSA at study entry was 0.9 ng/mL (range 0.25 – 17.60). This PSA range was considerably lower than in the registrational studies for the approved PSMA PET agents in BCR. Among the patients who did not complete the study as they proceeded to systemic therapy (which was not allowed according to the trial protocol), the majority had lesions identified on the 64Cu-SAR-bisPSMA scan, but they did not contribute towards the assessment of the efficacy endpoints. Results from COBRA showed for the first time that 64Cu-SAR-bisPSMA is safe and effective in detecting lesions in patients with BCR of PC who were negative or equivocal on SOC imaging (e.g. bone scan, CT or PET with approved PSMA imaging agents) at screening (Figures 1 and 2).

Figure 1. Identification of lesion in the pelvic region using 64Cu-SAR-bisPSMA on next-day imaging (right), negative on same-day imaging (64Cu-SAR-bisPSMA; center) and equivocal on screening SOC imaging (18F-DCFPyL, Pylarify®; left). SUVmax of the lesion across scans (arrows and red circles) was 2.3, 4.3 and 17.5 (18F-DCFPyL, Pylarify®, Day 0 and Day 1 64Cu-SAR-bisPSMA, respectively). Top images: PET/CT fusion. Bottom images: PET.
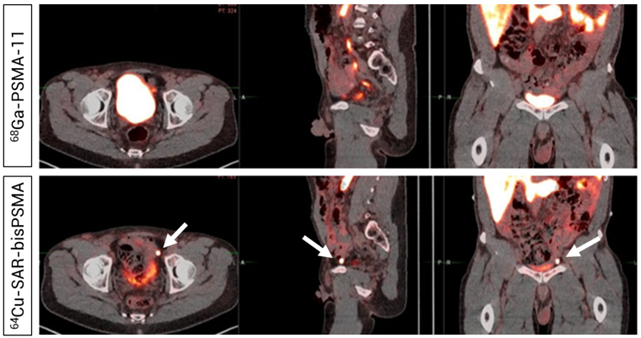
Figure 2. Extra-pelvic lesion identified on next-day imaging using 64Cu-SAR-bisPSMA on Day 1 but not detected on Day 0 (arrows). Top images: PET/CT fusion. Bottom images: PET.
Only one adverse event was related to 64Cu-SAR-bisPSMA (Grade 2 worsening of type II diabetes, resolved) among all 52 patients who received the product (Safety Analysis Set). 64Cu-SAR-bisPSMA identified lesions in up to approximately 60% of patients on same-day imaging, and up to 80% on next-day imaging, with high specificity on both days (Figure 3).
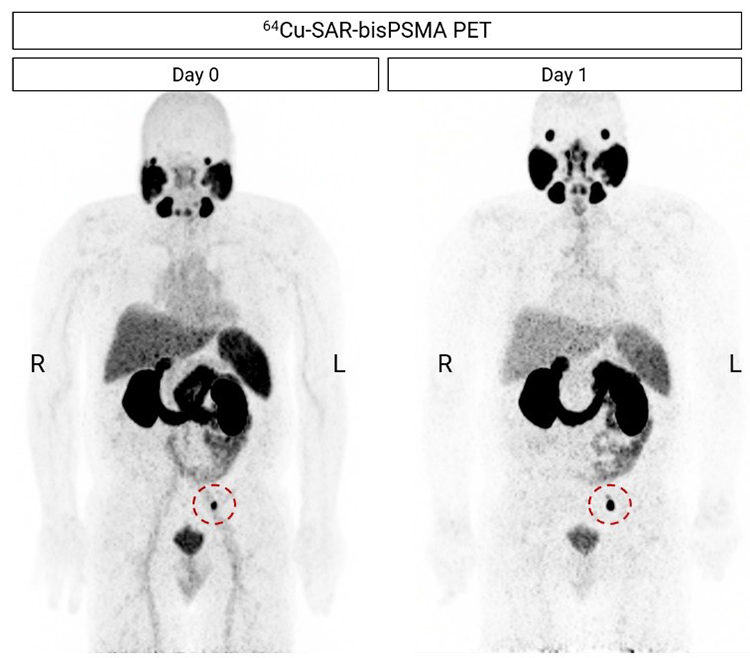
Figure 3. 64Cu-SAR-bisPSMA PET showing a positive pelvic lymph node (LN; red circle, maximum intensity projection). CT-guided needle biopsy of the lesion was performed and was negative for PC. This was followed by an excisional biopsy of the lesion, which confirmed the presence of PC by histopathology. Next-day imaging showed an increase of SUVmax of more than double compared to same-day imaging, from 20.8 on Day 0 to 50.4 on Day 1. All images are displayed at Maximum Intensity Projection.
The primary efficacy endpoints were patient-level correct detection rate (CDR, defined as the proportion of true positive participants out of all scanned participants who had at least one evaluable reference standard datapoint) and region-level positive predictive value (PPV, defined as the proportion of true positive regions out of all positive regions on the 64Cu-SAR-bisPSMA PET/CT scan with corresponding evaluable reference standard). The CDR range across the central readers on Day 0 was 21.4–28.6% (95% confidence interval [CI] 10.3–44.6), increasing to 28.6–38.1% (95% CI 15.7–54.4) on Day 1. The region-level PPV on Day 0 range was 39.1–44.8% (95% CI 19.7–64.3) and on Day 1 was 32.7–43.3% (95% CI 20.3–62.6). The specificity of PC detection in the pelvic LNs, a common site of PC metastases, was high across all readers and on both days (range across readers of 93.8–96.9% on Day 0 [95% CI 79.2–99.9] and 81.3–87.9% on Day 1 [95% CI 63.6–96.6]). The CDR and PPV results were substantially impacted by the large number of lesions that were detected, but unable to be biopsied as this would not be clinically appropriate. Similarly, the relatively modest decrease in specificity on Day 1 was also related to the challenges to obtain the reference standard for the considerably increased number of lesions identified on Day 1. The study had biopsies performed in only 9 patients, of whom 7 (almost 80%) had a positive result based on histopathology. One particular example shows the limitations even for the use of biopsies to validate the 64Cu-SAR-bisPSMA PET findings, where the first biopsy of a lymph node had a negative histopathology result for PC, which turned out positive following the complete excision of the same lymph node (Figure 3, above). The limited number of biopsies seen in the study coupled with the low sensitivity of the SOC imaging led to some lesions not being validated as true positives. Furthermore, among the patients who did not contribute to the efficacy endpoints assessment and had started systemic therapy while on study (not allowed according to the protocol), the majority had positive 64Cu-SAR-bisPSMA scans. This demonstrates the need for different study design approaches to assess the efficacy of innovative products which have additional benefits compared to those already available in the market.
Following 64Cu-SAR-bisPSMA PET imaging, investigators indicated that they would change the treatment plan of approximately half of the patients (48%). Of these patients, two-thirds (67%) proceeded to receive systemic and/or focal therapy. Taken altogether, the findings from the COBRA trial have important clinical implications as the localisation of recurrence may lead to different treatment pathways for these patients.
Next-day imaging – a key advantage
The possibility of performing next-day imaging is a feature not available to currently approved PSMA-targeted PET products and unique to 64Cu-based SAR diagnostics due to the optimal half-life of 64Cu and the ability of the SAR Technology to prevent leakage of copper isotopes from the radiopharmaceutical in-vivo. The COBRA trial confirmed the benefits of delayed imaging in this patient group as more lesions and more patients with a positive scan were identified on next-day imaging.
64Cu-SAR-bisPSMA was able to identify approximately 91% more lesions (median increase) on next-day imaging compared to same-day imaging (ranges across the readers for the total number of lesions: 53–80 on Day 0 vs. 82–153 on Day 1). The number of lesions in the pelvic LN region more than doubled on next-day imaging compared to same-day imaging (median increase of 108.3% across all readers comparing Day 0 vs. Day 1) (Figures 4 and 5). The ranges of CDR and patient-level detection rate (DR, defined as the proportion of participants with a positive 64Cu-SAR-bisPSMA PET/CT scan out of all scanned participants) were both higher on Day 1 compared to Day 0. The DR range on Day 0 was 44–58% (95% CI 30–71.8), increasing on Day 1 to 58–80% (95% CI 43.2–90).
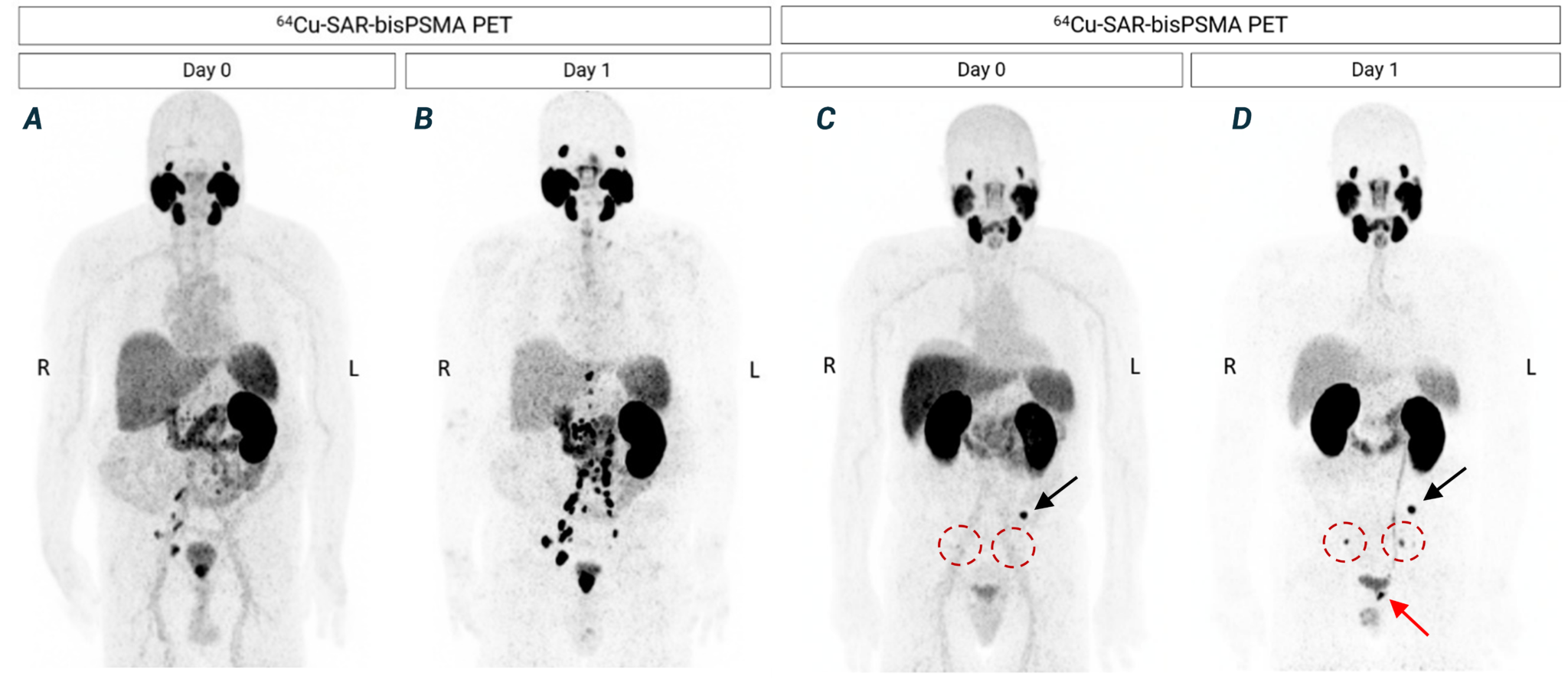
Figure 4. Next-day imaging identified additional lesions compared to same-day imaging. A, B – patient 1: 64Cu-SAR-bisPSMA PET showing positive LNs in the pelvic and extra-pelvic (retroperitoneal) regions and lesions in the prostatic bed. C, D – patient 2: 64Cu-SAR-bisPSMA PET showing a lesion in the pelvic bone (black arrow) on both days, pelvic LNs more clearly visualised on next-day image (red circles), and prostatic bed lesion only visualised on next-day imaging (red arrow). Average SUVmax for the bone and pelvic LN lesions increased from 9.8 on Day 0 to 20.0 on Day 1. PC in the pelvic LNs was confirmed by histopathology in both patients. All images are displayed at Maximum Intensity Projection.
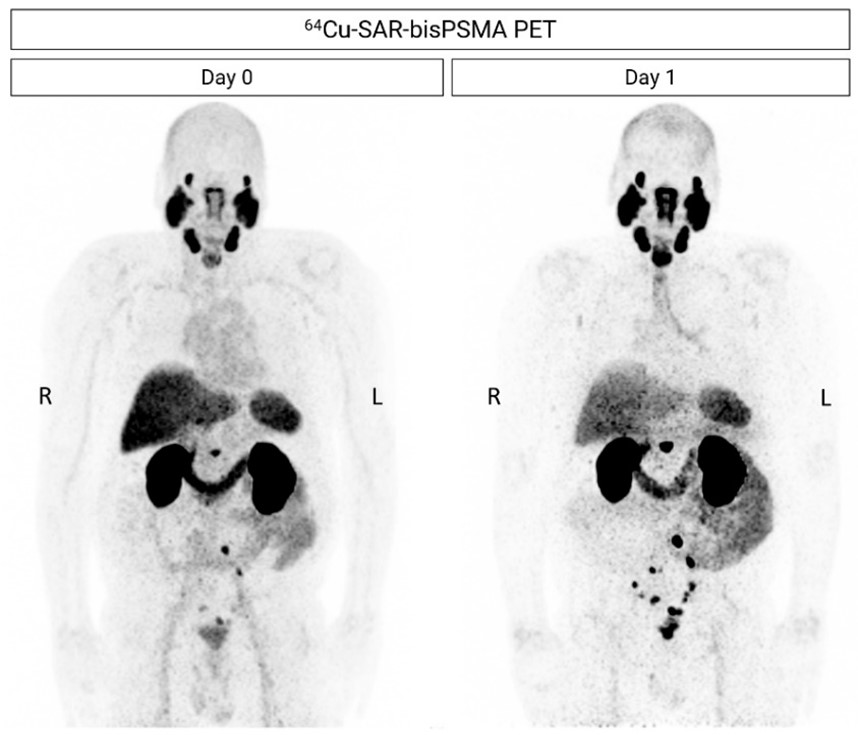
Figure 5. Next-day imaging identified additional lesions compared to same-day imaging. 64Cu-SAR-bisPSMA PET showing positive LNs in the pelvic, extra-pelvic (retroperitoneal) and prostatic bed regions. All images are displayed at Maximum Intensity Projection.
“These initial results from the COBRA study confirmed the advantages of the product not only by detecting lesions in patients whose SOC scans were negative or equivocal at screening, but also by identifying more lesions on next-day imaging compared to same-day scans. Such properties are unique to 64Cu-SAR-bisPSMA and demonstrate the ability of the product to identify lesions missed by other diagnostic imaging modalities with low sensitivity. As 64Cu-SAR-bisPSMA with the option of the next-day scan may represent a new paradigm in diagnostic imaging, the results of this trial are essential to inform potential innovative study designs for future investigations, including the design of the registrational Phase 3 study using the product in the BCR of PC setting, in which planning has commenced.
“We are thrilled to continue the development of SAR-bisPSMA in various stages of PC, as a standalone diagnostic product and also as a theranostic pair in our Targeted Copper Theranostic (TCT) platform. The PROPELLER study results originally showed the potential of SAR-bisPSMA in the pre-prostatectomy setting, whereby PC lesions had a 2-3 times greater uptake using 64Cu-SAR-bisPSMA (same-day imaging) compared to 68Ga-PSMA-11 (Figure 6). 64Cu-SAR-bisPSMA was also able to identify PC lesions not detected by 68Ga-PSMA-11 in these patients (Figure 7). The COBRA trial now formally expands the potential use of 64Cu-SAR-bisPSMA into the BCR of PC setting with the added benefit of next-day imaging, and it represents a step forward in bringing innovative products to patients in need of highly effective and safe diagnostic agents”, Dr Taylor commented.
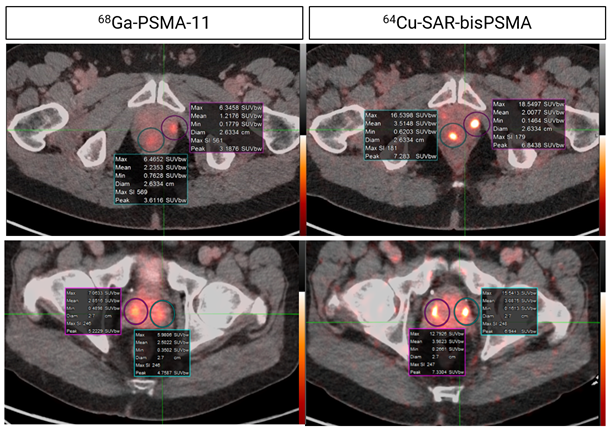
Figure 6. Concordant lesions on 64Cu-SAR-bisPSMA (200 MBq) and 68Ga-PSMA-11 PET/CT consistently showed higher SUVmax, SUVmean and TBR with 64Cu-SAR-bisPSMA compared to 68Ga-PSMA-11 in all cohorts (statistically significant values for all parameters). Interval between scans: top 8 days; bottom 35 days. Images: PET/CT fusion.
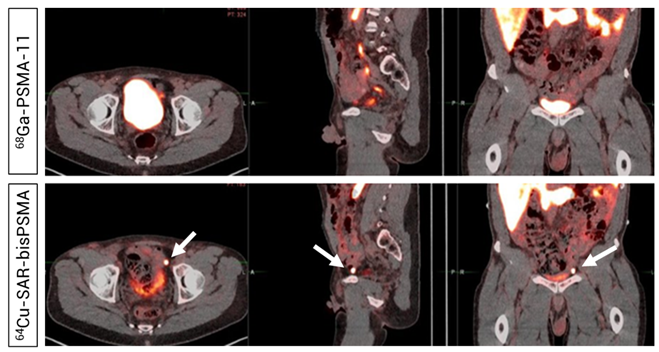
Figure 7. Readers did not detect uptake in pelvic lymph nodes on the 68Ga-PSMA-11 PET/CT (Top). PET/CT demonstrated uptake of 64Cu-SAR-bisPSMA (200 MBq, Bottom) in a left pelvic lymph node according to both Readers and PC was confirmed via histopathology. Arrows highlight the node detected on 64Cu-SAR-bisPSMA PET/CT. Interval between serial imaging: 7 days. Images: PET/CT fusion.
References:
- Clinicaltrials.gov Identifier: NCT05249127<https://clinicaltrials.gov/ct2/show/NCT05249127>
- Clinicaltrials.gov Identifier: NCT06056830, <clinicaltrials.gov/ct2/show/NCT06056830>
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, <https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660>
- American Cancer Society: Key Statistics for Prostate Cancer, <https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html>
Source: Clarity Pharmaceuticals. (2024). Initial COBRA Results: Clarity’s SAR-bisPSMA Is Safe and Highly Effective in Detecting Tumours in Prostate Cancer Patients. Phase 3 Planning Underway. [Press release]. https://www.claritypharmaceuticals.com/news/cobra_results/.


