Given the success of treatment with curative intent in this population, a major focus is now on preventing the potential side effects of treatment. For example, results of the Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (ASCENDE-RT) trial showed that between 20% and 31% of patients treated with radiotherapy still had gastrointestinal side effects at 5-years post treatment.4 Using a hydrogel spacer (Figure 1) to increase the perirectal separation distance has been shown to limit the radiation exposure of the rectum and reduce the incidence of late follow-up side effects.5 Resource constraints mean, however, that not all eligible patients can receive a hydrogel rectal spacer as part of their care.
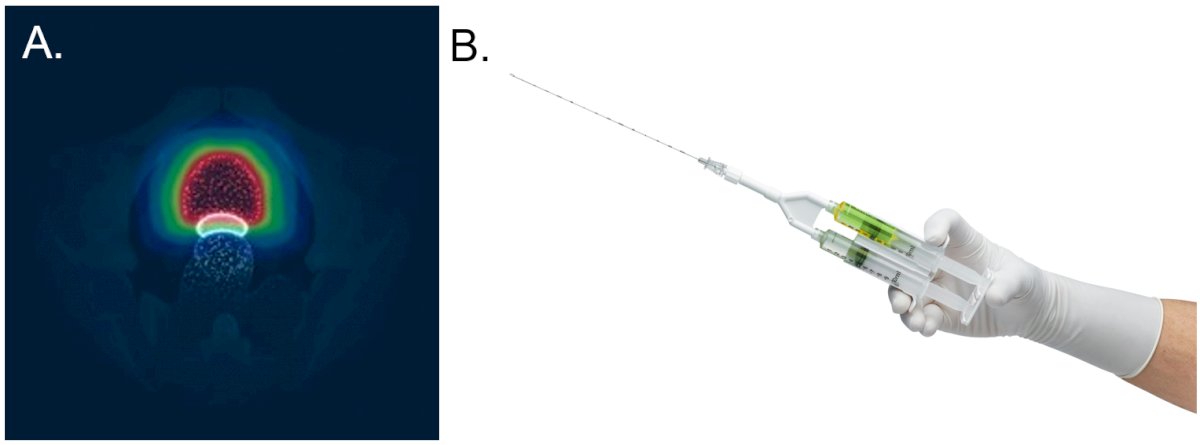
Figure 1. SpaceOar hydrogel rectal spacer: A, the SpaceOar hydrogel spacer (shown in white) is positioned between the prostate and the rectum. B, the hydrogel spacer is inserted using the syringe system shown.
The premise of this Delphi study was to build consensus on which patients in the UK should be prioritized to receive a rectal hydrogel spacer during prostate cancer treatment with radiotherapy. Our Delphi study used a five-stage approach to elicit consensus, consisting of two online questionnaires; a two-part, virtual meeting to discuss the questionnaire results; and a final concluding consensus questionnaire (Figure 2). Seven medical experts agreed to contribute to the Delphi panel, representing care provision across a wide range of the UK.
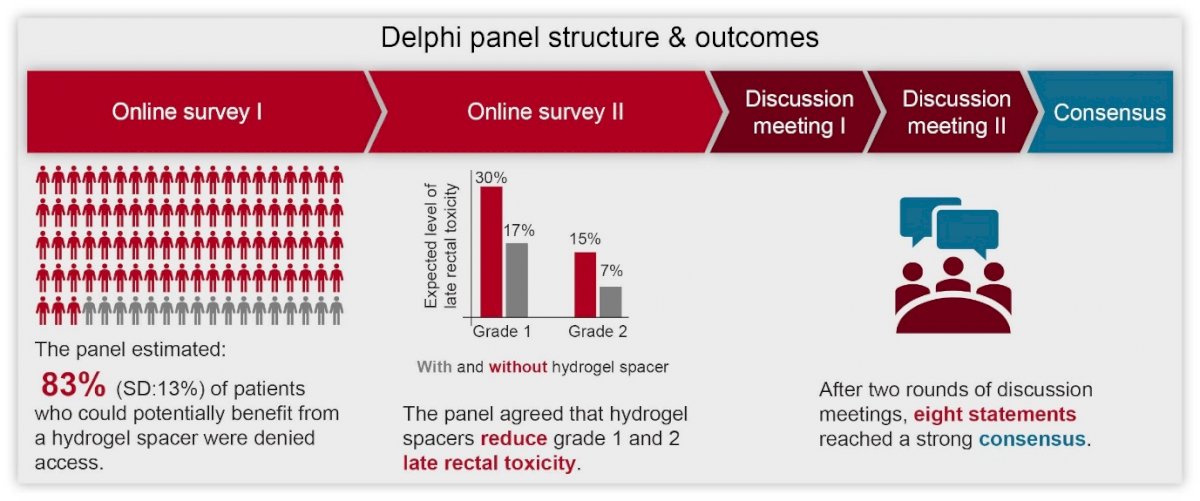
Figure 2. Overview of the Delphi process
Experts agreed that, when used appropriately, rectal spacers were expected to reduce the level of rectal toxicity patients experience. The size of the unmet need facing patients and providers was highlighted in the initial questionnaire, with the experts estimating that on average 83% (SD: 13%) of patients who could potentially benefit from a hydrogel spacer were currently denied access. Discussion on how to prioritize patients for access to a rectal spacer considered both patient and treatment characteristics. Although no single system to identify patients most likely to benefit from a rectal spacer was identified, the expert panel did reach strong consensus on eight statements. It was their consensus opinion that:
- for treatments with curative intent, focus should be on minimising toxicity and the risk of side effects.
- use of spacers in eligible patients significantly reduces radiation dose to the rectum and toxicity-related adverse events.
- despite meeting rectal dose constraints, too many patients continue to experience rectal toxicity.
- certain grade one toxicity-related adverse events (bowel frequency and urgency, diarrhoea, flatulence, radiation cystitis, radiation proctitis, rectal bleeding and rectal mucus) can still have a significant impact on patient quality of life.
- any toxicity grading system in use should be complemented by patient-reported outcomes.
- patients receiving long-term anticoagulation therapy with medications such as direct oral anticoagulants (DOACs) (The reason for prescribing the DOAC, rather than the medication itself, is more important for the decision. All patients on DOACs, except for cardiac stent and prosthetic valve replacement patients, may be able to safely pause their anticoagulation.) should be considered for spacer use if their anticoagulation can be safely paused.
- spacers are useful in eligible patients with T1-T2 disease. Spacer use in patients with T2+ disease should not be excluded but should be assessed on an individual basis by a team proficient in inserting spacers.
- patients should have the opportunity to take part in the discussion regarding the use of a spacer.
Written by: Rhodri Saunders,1 Heather Payne,2 Suneil Jain,3 Clive Peedell,4 Albert Edwards,5 Andrew Thomas,6 Prantik Das,7 Emily Woodward,8 Amit Bahl9
- Coreva Scientific GmbH und Co KG, Königswinter, Germany
- Department of Oncology, University College London Hospitals NHS Foundation Trust, London, UK
- School of Medicine, Dentistry and Biomedical Sciences, Queen's University Belfast, Belfast, UK
- Department of Radiotherapy and Oncology, James Cook University Hospital, Middlesbrough, UK
- Kent Oncology Centre, Kent, UK
- Department of Urology, Princess of Wales Hospital, Bridgend, UK
- Department of Oncology, University Hospitals of Derby and Burton NHS Foundation Trust, Derby, UK
- Health Economics, Boston Scientific AG, Solothurn, Switzerland
- Bristol Haematology and Oncology Centre, University Hospitals Bristol NHS Foundation Trust, Bristol, UK
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. Cancer Research UK
- Prostate cancer statistics. Available: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Zero [Accessed 23 Nov 2021].
- Prostate Cancer UK . Prostate information. About prostate cancer 2019. Available: https://prostatecanceruk.org/prostate-information/about-prostate-cancer [Accessed 23 Nov 2021].
- Rodda S, Tyldesley S, Morris WJ, et al. ASCENDE-RT: an analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:286–95
- Miller LE, Efstathiou JA, Bhattacharyya SK, Payne HA, Woodward E, Pinkawa M. Association of the Placement of a Perirectal Hydrogel Spacer With the Clinical Outcomes of Men Receiving Radiotherapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020 Jun 1;3(6):e208221. doi: 10.1001/jamanetworkopen.2020.8221
Read the Abstract


