Although sharing the identical urea-binding motif (Figure 1, red), the so-called linker (blue) and chelator (green) required for labeling with Lutetium-177 are different. In this regard, a DOTA chelator is used for [177Lu]Lu-PSMA-617,6 while for synthesis of [177Lu]Lu-PSMA I&T a DOTAGA chelator is required.7 Despite their structural differences, a recent dosimetry study indicated that the both compounds are comparable in their efficacy and safety.8 Urologists and nuclear medicine specialists at the University Hospitals Bonn and Würzburg (both Germany) therefore pooled data on 110 metastatic castration-resistant PC patients treated with both compounds.
After carefully adjusting for various relevant clinical factors (including age, Gleason score, PSA levels, and prior chemotherapy), such a matched-pair comparison demonstrated that the rate of clinically relevant toxicities was low for both agents. In fact, only five grade III anemia for [177Lu]Lu-PSMA-617 and one thrombopenia were recorded ([177Lu]Lu-PSMA I&T, one grade III anemia). In addition, median overall survival was comparable for both groups ([177Lu]Lu-PSMA I&T, 12 months vs. [177Lu]Lu-PSMA-617, 13 months).
As the most frequently applied agents for RLT to date, the present study indicates that those compounds can be used interchangeably, and thus, the referring urologist can have certainty that both urea-based small molecules achieve equivalent outcome benefits along with low toxicity profiles.
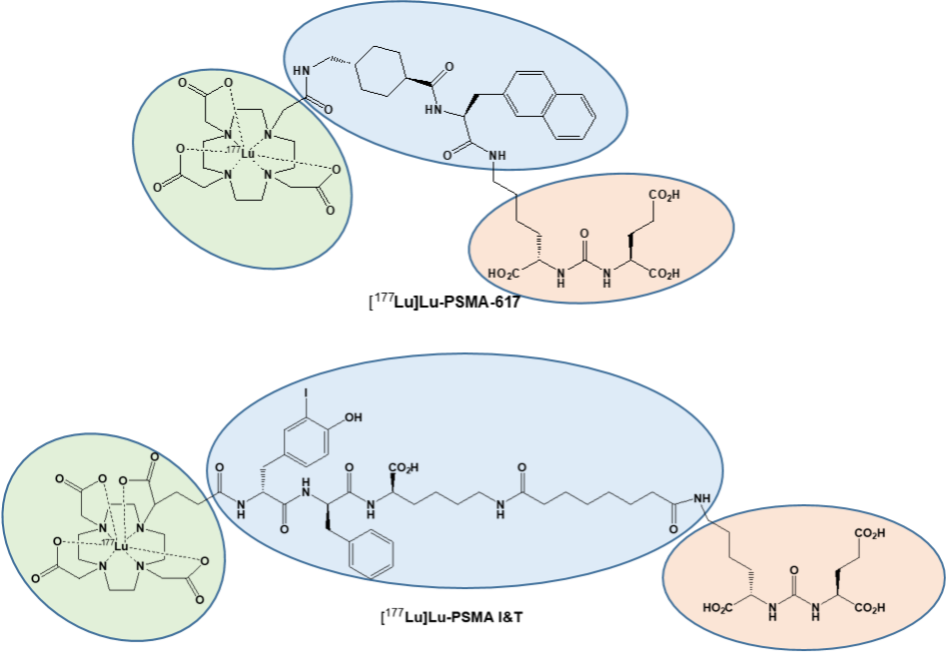
Figure 1: Chemical structures of [177Lu]Lu-PSMA I&T and [177Lu]Lu-PSMA-617. Modified from Hartrampf et al, Eur J Nucl Med Mol Imaging, 2022 Mar 4. doi: 10.1007/s00259-022-05744-6.
Written by: Philipp E. Hartrampf,1 Franz-Xaver Weinzierl,1 Andreas K. Buck,1 Steven P. Rowe,2 Takahiro Higuchi,1 Anna Katharina Seitz,3 Hubert Kübler,3 Andreas Schirbel,1 Markus Essler,4 Ralph A. Bundschuh,4 and Rudolf A. Werner1
- Department of Nuclear Medicine, University Hospital Wuerzburg, Oberdürrbacherstraße 6, 97080 Würzburg, Germany;
- The Russell H Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, 601 N Caroline Str, Baltimore, MD;
- Department of Urology and Paediatric Urology, University Hospital Wuerzburg, Oberdürrbacherstraße 6, 97080 Würzburg, Germany;
- Department of Nuclear Medicine, University Hospital Bonn, Venusberg-Campus 1, 53127 Bonn, Germany.
- Calais J, Gafita A, Eiber MR, Armstrong WR, Gartmann J, Thin P, et al. Prospective phase 2 trial of PSMA-targeted molecular RadiothErapy with (177)Lu-PSMA-617 for metastatic Castration-reSISTant Prostate Cancer (RESIST-PC): Efficacy results of the UCLA cohort. J Nucl Med. 2021. doi:10.2967/jnumed.121.261982.
- Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825-33. doi:10.1016/s1470-2045(18)30198-0.
- Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang SP, Kong G, et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of (177)Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J Nucl Med. 2020;61:857-65. doi:10.2967/jnumed.119.236414.
- Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet (London, England). 2021;397:797-804. doi:10.1016/s0140-6736(21)00237-3.
- Sadaghiani MS, Sheikhbahaei S, Werner RA, Pienta KJ, Pomper MG, Solnes LB, et al. A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177-labeled Prostate-specific Membrane Antigen-targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Eur Urol. 2021;80:82-94. doi:10.1016/j.eururo.2021.03.004.
- Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J Nucl Med. 2015;56:914-20. doi:10.2967/jnumed.114.147413.
- Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J Nucl Med. 2015;56:1169-76. doi:10.2967/jnumed.115.158550.
- Schuchardt C, Zhang J, Kulkarni HR, Chen X, Mueller D, Baum RP. Prostate-specific membrane antigen radioligand therapy using (177)Lu-PSMA I&T and (177)Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: comparison of safety, biodistribution and dosimetry. J Nucl Med. 2021. doi:10.2967/jnumed.121.262713.
Read the Abstract


