The premise of the ICE-PAC study was to investigate whether the administration of stereotactic ablative body radiotherapy (SABR) could enhance the anti-tumour activity of PD-L1 inhibitor blockade in patients with mCRPC. Preclinical studies have demonstrated the unique effects of ionizing radiation on the tumour microenvironment, including optimization of neoantigen formation, induction of proinflammatory cytokines and increased migration of tumour-specific effector T cells.5 High-dose radiotherapy in the form of SABR has been shown to be potentially synergistic with immune checkpoint inhibitors, but to date, clinical evidence of this phenomenon in mCRPC is lacking.
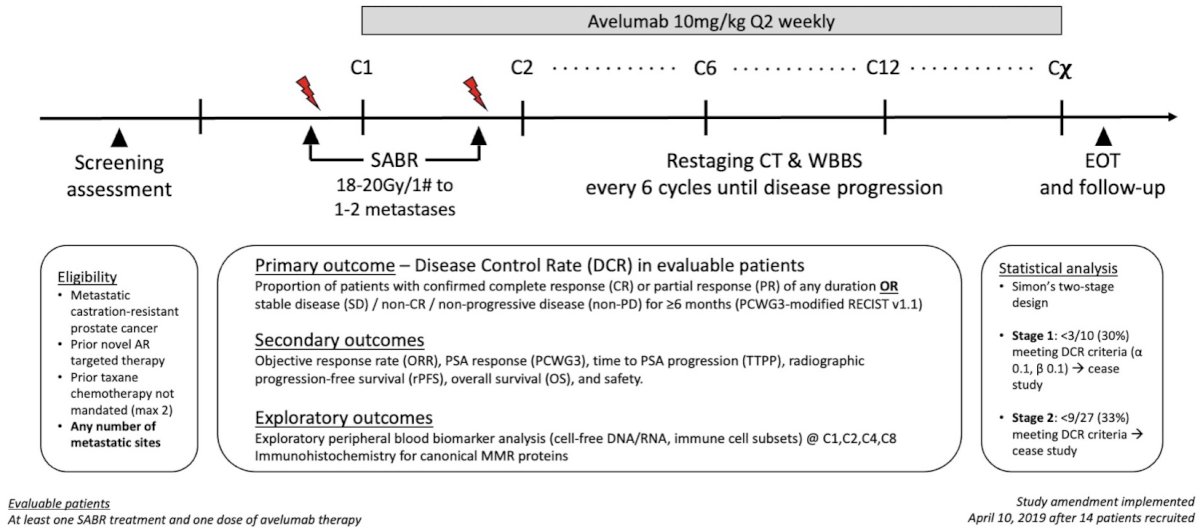
Figure 1: ICE-PAC study design
ICE-PAC was a single-arm, phase II Australian study of PD-L1 inhibitor avelumab combined with SABR (Fig. 1). SABR was administered within five days of cycle 1 and cycle 2 of avelumab infusion. Initially, avelumab was limited to 12 cycles, but a subsequent protocol amendment permitted avelumab until disease progression. The primary endpoint was disease control rate, defined as the proportion of patients with confirmed complete response (CR)/partial response (PR) of any duration, or stable disease (SD)/non-CR/non-PD for ≥6 months as per PCWG3-modified RECIST. Secondary endpoints included objective response rate, PSA response, radiographic progression-free survival (PFS), overall survival (OS), and safety. In this signal-seeking study, a Simon two-stage design with futility assessment was adopted.
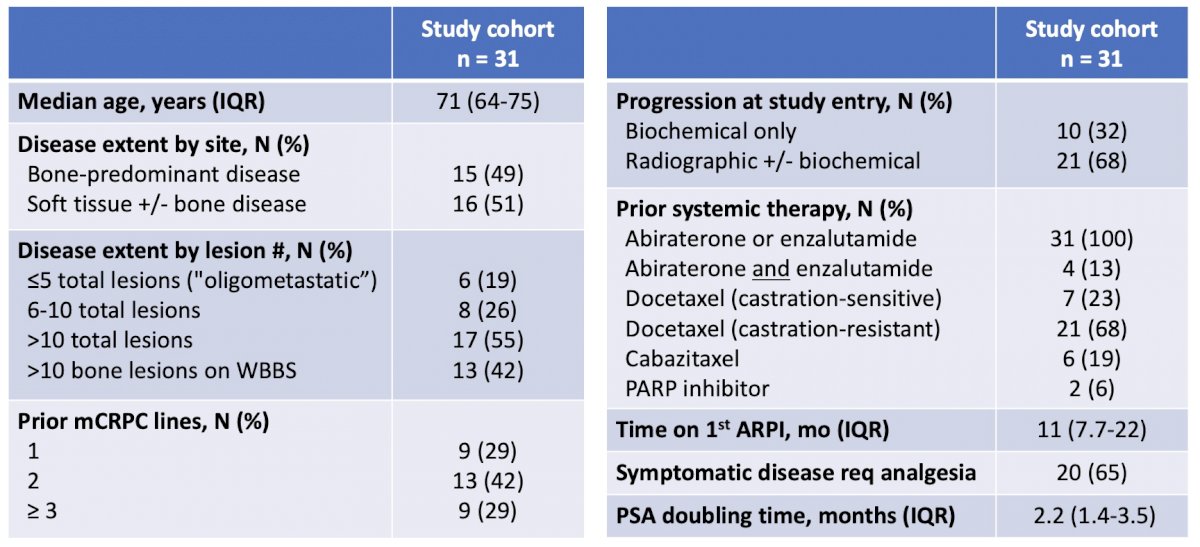
Table 1: Patient characteristics
The study recruited 31 evaluable patients assessable for the primary endpoint. In general, this was a heavily pretreated and poor-risk cohort. All patients had received prior second-generation AR-targeted therapy, 84% had prior taxane exposure, <20% exhibited oligometastatic disease (≤ 5 total lesions) and most were symptomatic and/or radiographically progressing at study entry (Table 1). A total of 70 lesions received SABR treatment, with a vast majority administered to bone and lymph node sites. Of note, since >80% of patients had polymetastatic disease (> 5 total lesions), the goal of delivering SABR was not to ablate all or even most sites of disease, but rather to function as an “immune-priming” modality.
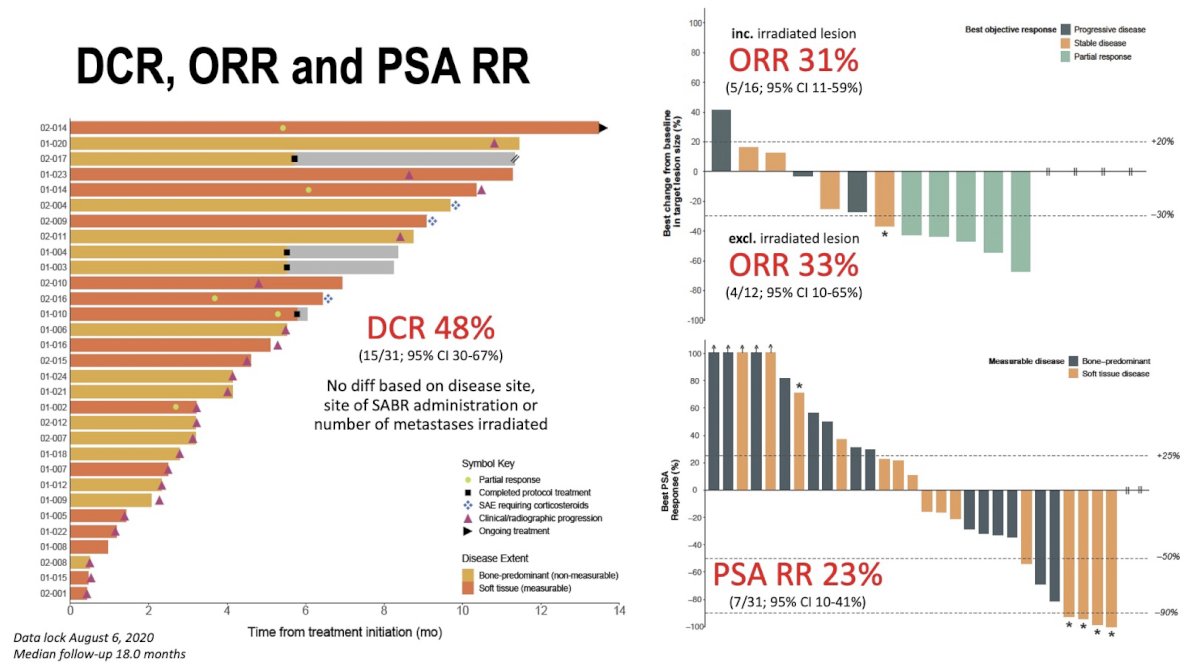
Figure 2: Disease control, overall response and PSA response
After a median follow-up of 18 months, the ICE-PAC study met its primary endpoint (Fig. 2). DCR was 48%, with no difference based on disease site, site of SABR administration, or number of metastases irradiated. Intriguingly, up to one-third experienced radiographic response, even when excluding lesions that received SABR, perhaps reflecting an abscopal response. Radiographic PFS and OS were 8.4 and 14.1 months, respectively, comparing favourably to prior monotherapy studies. Toxicity was acceptable, with only 10% discontinuing treatment because of adverse events. Collectively, these findings show that avelumab with SABR is an active and safe combination warranting further investigation in larger cohorts.
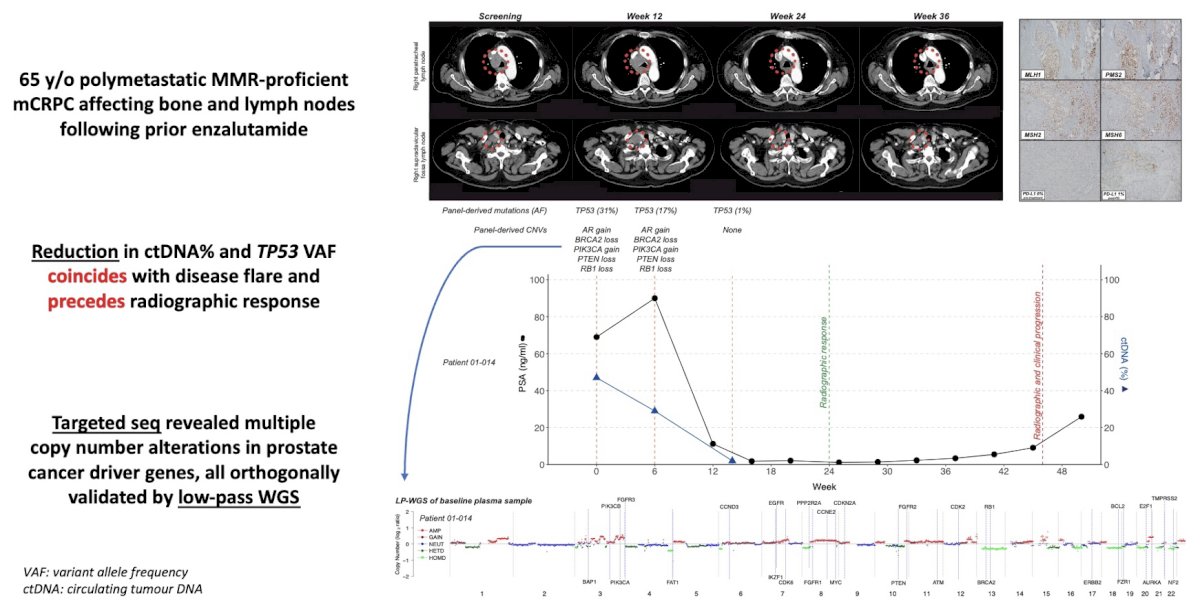
Figure 3: Molecular analysis of cell-free DNA/RNA and immunohistochemistry in a participant on the ICE-PAC trial.
We also performed exploratory molecular biomarker analysis on a subgroup of patients with available baseline and on-treatment peripheral blood samples. Using combined targeted sequencing and low-pass whole genome sequencing of plasma cell-free DNA (cfDNA)/RNA (cfRNA), we showed that i) specific alterations in AR and MYC genes were associated with poor outcomes, ii) serial cfDNA/RNA profiling may predict benefit from combination avelumab and SABR before biochemical and radiographic features (Fig. 3), and iii) candidate resistance mechanisms to immune checkpoint inhibitors plus SABR include neuroendocrine dedifferentiation and cumulative copy number alterations, particularly in the AR gene and tumour suppressors.
In conclusion, ICE-PAC is the first study to evaluate the combination of stereotactic radiotherapy and PD-1/PD-L1 pathway inhibition in mCRPC. We showed that SABR + Avelumab was well tolerated and associated with highly encouraging disease control rates, including in non-irradiated lesions (abscopal response). These data provide strength to the idea that SABR may be synergistic with immune checkpoint inhibitors, and will serve as a framework for designing future, prospective randomized clinical trials. Finally, these data add to mounting evidence for the utility of liquid biopsy approaches to monitor response and resistance in advanced prostate cancer.
Written by: Edmond M Kwan,1 Lavinia Spain,2 Angelyn Anton,3 Ben Tran,2 Shankar Siva,4 Arun A Azad2
- Department of Medicine, School of Clinical Sciences, Monash University, Melbourne, Australia
- Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, Australia
- Department of Medical Oncology, Eastern Health, Melbourne, Australia
- Division of Radiation Oncology, Peter MacCallum Cancer Centre, Melbourne, Australia
References:
- Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 700–712.
- Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol 2020; 38: 395–405.
- Fakhrejahani F, Madan RA, Dahut WL, et al. Avelumab in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2017; 35: 159–159.
- Sharma P, Pachynski RK, Narayan V, et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020; 38: 489–499 e3.
- Turgeon GA, Weickhardt A, Azad AA, et al. Radiotherapy and immunotherapy: a synergistic effect in cancer care. Med J Aust 2019; 210: 47–53.
Funding support:
- Edmond M Kwan: NHMRC Postgraduate Scholarship, Cancer Council Victoria Postdoctoral Fellowship.
- Shankar Siva: Cancer Council Victoria Colebatch Fellowship.
- Arun A Azad: NHMRC project grant, Victorian Cancer Agency Clinical Research Fellowship, Astellas Investigator-initiated Grant.


