Another variable that is often mentioned but not previously quantified is the effect of patient cohort. Most studies evaluating mpMRI performance compare it to either radical prostatectomy histopathology or standard biopsy histopathology. Each cohort, however, carries its own biases. While whole mount histopathology from radical prostatectomy specimens allows pixel-by-pixel assessment for the presence of cancer, these cohorts have 100% prevalence of cancer and are biased towards higher-grade disease. On the other hand, while studies using TRUS biopsy histopathology include many men without cancer and thereby better represent the cancer prevalence in the target population, the measurement of mpMRI diagnostic accuracy based on biopsy as the gold standard is impaired by high rates of false-negative results in TRUS-biopsy.8
In our study, we addressed the impact of cohort selection bias on apparent MRI accuracy. We did this by retrospectively comparing the diagnostic accuracy of mpMRI in a biopsy cohort against a radical prostatectomy cohort, stratified by PIRADS score.
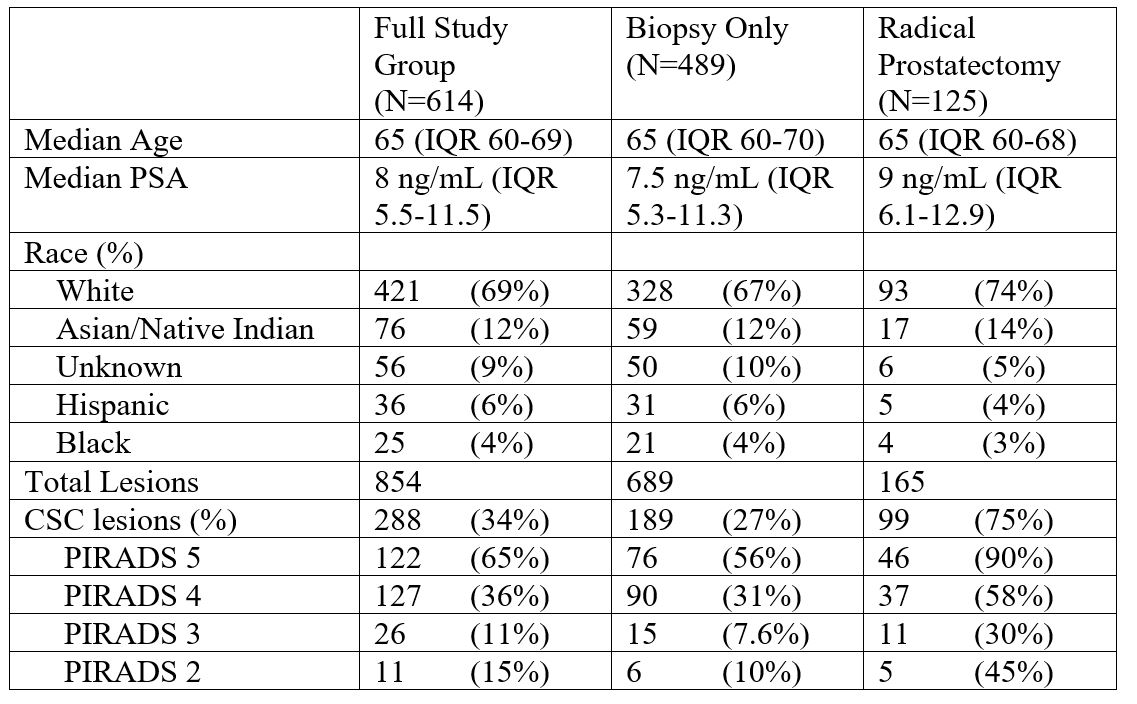
We found that for each PIRADS score, mpMRI detection of clinically significant cancer was higher in men who underwent radical prostatectomy than those who underwent biopsy only. While this finding is intuitive, it is important to recognize and quantify (Table 1). For example, cancer is present in 58% of PIRADS 4 lesions in the prostatectomy population, but only 31% of PIRADS 4 lesions in the biopsy population. This finding has important clinical implications. Clinicians should take great caution in counseling a man with an elevated PSA and abnormal MRI considering biopsy using data derived from a study using prostatectomy pathology as the gold standard. Instead, we advise using data from studies of more representative cohorts such as men without known cancer undergoing conventional biopsy, targeted biopsy, or perineal template mapping biopsy9.
Table 1: Demographic, clinical and biopsy results for men who underwent multiparametric MRI and targeted biopsy, separated into a biopsy-only cohort and radical prostatectomy cohort
Written by: Nancy N Wang, MD MPH1, Geoffrey A Sonn, MD1
1. Department of Urology, Stanford University School of Medicine, Stanford, California
References:
1. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, Briganti A, Budaus L, Hellawell G, Hindley RG, Roobol MJ, Eggener S, Ghei M, Villers A, Bladou F, Villeirs GM, Virdi J, Boxler S, Robert G, Singh PB, Venderink W, Hadaschik BA, Ruffion A, Hu JC, Margolis D, Crouzet S, Klotz L, Taneja SS, Pinto P, Gill I, Allen C, Giganti F, Freeman A, Morris S, Punwani S, Williams NR, Brew-Graves C, Deeks J, Takwoingi Y, Emberton M, Moore CM, PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–1777.
2. Mertan FV, Greer MD, Shih JH, George AK, Kongnyuy M, Muthigi A, Merino MJ, Wood BJ, Pinto PA, Choyke PL, Turkbey B. Prospective evaluation of the prostate imaging reporting and data system version 2 for prostate cancer detection. J Urol. 2016;196(3):690–696.
3. Lim CS, Mcinnes MDF, Lim RS, Breau RH, Flood TA, Krishna S, morash C, Shabana WM, Schieda N. Prognostic value of prostate imaging and data reporting system (PI-RADS) v. 2 assessment categories 4 and 5 compared to histopathological outcomes after radical prostatectomy. J Magn Reson Imaging. 2017;46(1):257–266.
4. Sonn GA, Fan RE, Ghanouni P, Wang NN ,Brooks JD, Loening AM, Daniel BL, To’o KJ, Thong AE, Leppert JT. Prostate magnetic resonance imaging interpretation varies substantially across radiologists. Eur Urol Focus. Epub 2017 Dec 06.
5. Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR. 2012;199:103-110.
6. Distler FA, Radtke JP, Bonekamp D, Kesch C, Schlemmer H-P, Wieczorek K, Kirchner M, Pahernik S, Hohenfellner M, Haschik BA. The value of PSA density in combination with PI-RADS for the accuracy of prostate cancer prediction. J Urol. 2017 Sep;198(3):575-82.
7. Gielchinsky I, Scheltema MJ, Cusick T, Chang J, Shnier R, Moses D, Delprado W, Nguyen Q, Yuen C, Haynes AM, Stricker PD. Reduced sensitivity of multiparametric MRI for clinically significant prostate cancer in men under the age of 50. Res Rep Urol. 2018;10:145-150.
8. Serefoglu EC, Altinova S, Ugras NS, Akincioglu E, Asil E, Balbay MD. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J. 2013 May-Jun;7(5-06):E293-8.
9. Ahmed HU, Bosaily AES, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, Kirkham AP, Oldroyd R, Parker C, Emberton M, PROMIS study group. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815-22
Read the Abstract


