However, most studies found no survival benefit for regular follow-up in colorectal cancer [1, 2], breast cancer [3], endometrial cancer [4], or lung cancer [5]. Similarly, it continues to be debated whether a routine oncological follow-up to detect asymptomatic recurrence after radical cystectomy (RC) improves patient survival [6-9]. Currently, only four studies have investigated the benefit of routine oncological follow-up; their conclusions were controversial. In a series of 1,270 patients who underwent RC, Volkmer et al. [6] found no overall survival advantage in those with asymptomatic vs. symptomatic tumor recurrence.
On the other hand, Giannarini et al. [7] noted that of 479 patients who underwent RC with orthotopic ileal neobladder reconstruction, those diagnosed with asymptomatic recurrence during routine follow-up had significantly improved cancer-specific and overall survival compared to patients diagnosed after symptomatic relapse. Boorjian et al. [8] reported the prognostic benefit in detecting asymptomatic recurrence in a series of 1,599 patients who underwent RC. More recently, Alimi et al [9] reported the prognostic benefit in detecting asymptomatic recurrence in a series of 331 patients who underwent RC (Table 1). Therefore, those conflicting evidences resulted in unconsolidated recommendation for surveillance and in a low rate of adherence to surveillance guidelines.
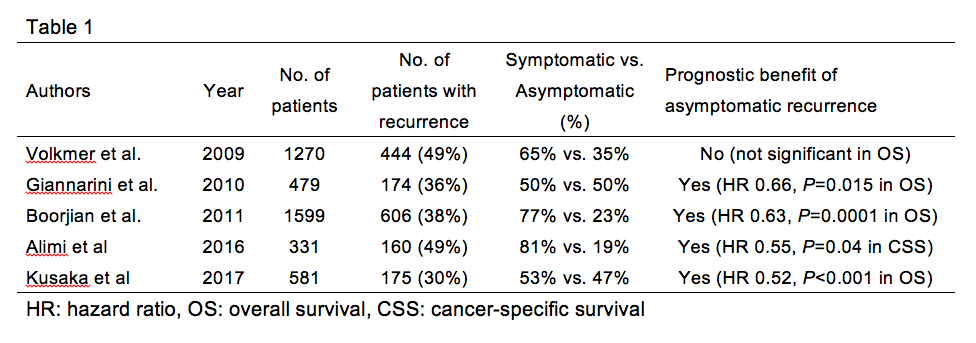
We retrospectively analyzed 581 RC cases for muscle invasive bladder cancer at four hospitals between May 1996 and February 2017. Of the 581 patients, 175 (30%) experienced relapse. At the time of diagnosis, 47% were asymptomatic and 53% were symptomatic. A multivariate Cox regression analysis using inverse probability of treatment weighting (IPTW) showed that in the patients with symptomatic recurrence was an independent risk factor for overall survival after RC (P < 0.001, HR: 1.94, 95% CI: 1.38–2.72) and survival from recurrence to death (P < 0.001, HR: 2.18, 95% CI: 1.55–3.08) [10]. Our results support the idea that an effective follow-up protocol following curative surgery should detect recurrence in the early stages of the disease and that detection with asymptomatic recurrence should secure sufficient time to implement a multimodal therapy after relapse.
Although the detection of asymptomatic recurrence contributed to better oncological outcomes after RC, there have been grave concerns that not only we detect the recurrence earlier, but just identifying the difference between rapid and slow growing diseases. Obvious tumor progression that is missed during routine follow-up suggests the existence of biological heterogeneity between rapid- and slow-growing tumors that could not have been detected by a conventional pathological examination. For a better understanding of tumor biology, not only the mode of diagnosis (asymptomatic vs. symptomatic) at recurrence but also biomarkers that predict the malignant potential are essential. There is an urgent need for the development of a biology-based molecular biomarker for the classification of bladder cancer to inform clinical management.
Cost-effectiveness is the other important issue for regular surveillance. The more we screen, the more we increase medical cost. On the other hand, the fewer we screen, the more we lost the change for therapy. Currently, no cost-effective surveillance protocol after RC is available. Now, we are trying to develop a risk-stratified surveillance protocol that reduce over-evaluation after RC without adverse effects on medical cost. We believe our next study provides the idea for risk stratification to improve the cost-effectiveness. A prospective study on the cost effectiveness of follow-up using a universal, standard, and easily applicable surveillance model is needed.
Written By: Shingo Hatakeyama, MD.
Read the Abstract
References
[1] Virgo KS, Vernava AM, Longo WE, McKirgan LW, Johnson FE. Cost of patient follow-up after potentially curative colorectal cancer treatment. JAMA. 1995;273:1837-41.
[2] Jeffery M, Hickey BE, Hider PN, See AM. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:CD002200.
[3] Rojas MP, Telaro E, Russo A, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2005:CD001768.
[4] Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006;101:520-9.
[5] Younes RN, Gross JL, Deheinzelin D. Follow-up in lung cancer: how often and for what purpose? Chest. 1999;115:1494-9.
[6] Volkmer BG, Schnoeller T, Kuefer R, Gust K, Finter F, Hautmann RE. Upper urinary tract recurrence after radical cystectomy for bladder cancer--who is at risk? J Urol. 2009;182:2632-7.
[7] Giannarini G, Kessler TM, Thoeny HC, Nguyen DP, Meissner C, Studer UE. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-94.
[8] Boorjian SA, Tollefson MK, Cheville JC, Costello BA, Thapa P, Frank I. Detection of asymptomatic recurrence during routine oncological followup after radical cystectomy is associated with improved patient survival. J Urol. 2011;186:1796-802.
[9] Alimi Q, Verhoest G, Kammerer-Jacquet SF, et al. Role of routine computed tomography scan in the oncological follow up of patients treated by radical cystectomy for bladder cancer. Int J Urol. 2016;23:840-6.
[10] Kusaka A, Hatakeyama S, Hosogoe S, et al. Detecting asymptomatic recurrence after radical cystectomy contributes to better prognosis in patients with muscle-invasive bladder cancer. Med Oncol. 2017;34:90.


