Aside from BCG-relapsing and BCG-refractory or intolerant patients, the global scarcity of BCG has been aggravating this situation even more. One promising antitumor strategy is to modulate the tumor microenvironment (TME) using innate immunity modulators, such as Toll-like Receptor (TLR) agonists.3 TLRs stimulation can activate both innate and adaptive immune responses, enhancing the recruitment and the activity of antitumor effector cells into the TME.4
OncoTherad® or Biological Response Modifier - Inorganic Phosphate Complex 1 (MRB-CFI-1, for its acronym in Portuguese) is an immunotherapy with patents granted in Brazil (BR102017012768B1), the USA (US11572284B2, US11136242B2), and Europe (EP3626746B1). OncoTherad® comprises phosphate and metal salts (CFI-1) linked to glycosidic proteins (P14 and P16 proteins) with a size of around 477.1 nm (one of its dimensions is smaller than 100nm).5 This nanostructured immunotherapy promotes local activation of the immune system and exhibits antitumor effects mainly through TLR4-mediated interferon (IFN) induction.6 A clinical trial testing OncoTherad® for bladder cancer is currently ongoing (Brazilian Clinical Trials Registry -RBR-6swqd2, UTN U1111-1226–9096).7 In the field of unexplored modulators, platelet-rich plasma (PRP) has been increasing in having immune-modulation properties reported.8,9 The research team responsible for the OncoTherad® development (Laboratory of Urogenital Carcinogenesis and Immunotherapy, LCURGIM/ University of Campinas, Brazil) also reported that PRP can be used in the context of bladder cancer even associated with BCG in pre-clinical models.10
In our study, we employed OncoTherad® as a single strategy and in combination with PRP to treat non-muscular invasive bladder cancer (NMIBC) using a well-established mice model.11 OncoTherad® stimulated both interleukins (canonical) and interferons (non-canonical) signaling pathways mainly through TLR4, promoting significant increases of NF-kB, IL-6, IL-1β, TBK-1, and IFN-γ. Although PRP did not reduce neoplastic lesions as a single treatment, it still had effects on the non-canonical pathway by increasing TLR4, IFN-γ, and NF-kB levels. Interestingly, OncoTherad® associated with PRP stimulated TLR4 even more intensely - compared not only with the cancer therapy-free group but also with the isolated treatments. Moving forward, the stimulation of immune pathways for IFN-γ production modulated the NMIBC microenvironment to a cytotoxic profile, characterized by an increase in IL-1β, activation of cytotoxic T lymphocytes (as CD8+ T-cells), and reduction in regulatory T-cells (Tregs).
We need to point out that the OncoTherad® therapeutic dose (20 mg/ml) in the combined strategy was reduced by half (10 mg/ml) and promoted a statistically equivalent tumor regression rate compared to when it was used as a single therapy (71.4% vs. 85.7%). This showed PRP playing a role in treating induced bladder cancer when associated with immunotherapy. It is known that platelets' role in innate and adaptive immunity is related to the action of growth factors and interactions with leukocytes and endothelial cells.8 The physicochemical characterizations indeed showed a higher concentration of PRP proteins upon interaction with OncoTherad® - the phosphates present in the inorganic part of the immunotherapy induced the release of proteins by platelets. Further, both human and murine platelets can express TLRs such as TLR2 and TLR4, for which OncoTherad® acts as an agonist.8 TLR4 signaling in platelets mediates TNF-α production by leukocytes and activation of the innate immune system. Last but not least, although PRP contains growth factors, the use of intravesical PRP did not increase VGF-1 and IGF (progression biomarkers) nor favored bladder carcinogenesis.
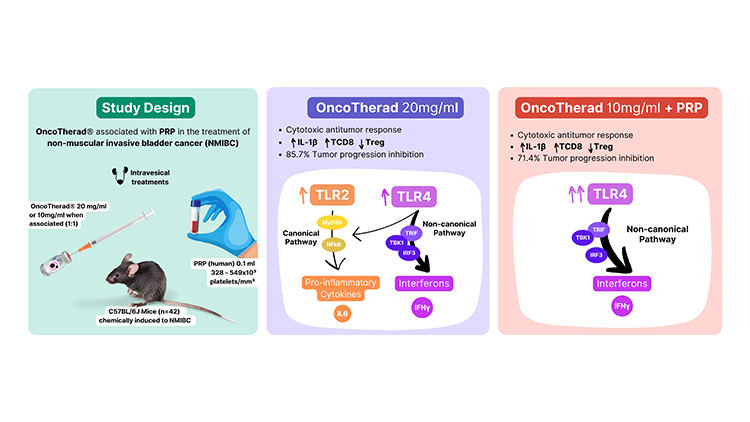
In summary, PRP modulated and intensified the OncoTherad® stimulation on the TLR4-mediated non-canonical pathway, allowing a lower dose of the immunotherapy to achieve the same antitumor effect. This new therapeutic strategy addresses personalization and reduction of immunogenicity - since PRP is obtained from the patient's blood - as well as management of the immunomodulation balance - which can be responsible for the immunotherapies' side effects related to immune overstimulation. There is for sure a long pathway ahead to address the unmet needs of HRNMIBC patients. While OncoTherad® is emerging as a new option for BCG-unresponsive patients, this study reinforces its promising immunomodulatory properties and represents a step forward in the development of new therapeutic approaches in bladder cancer.12–15
Written by: Bianca Ribeiro de Souza, PhD1,2
- Faculty of Medicine, Department of Obstetrics and Gynecology, University of British Columbia, Vancouver General Hospital, Vancouver, BC, Canada
- Department of Structural and Functional Biology, Institute of Biology – University of Campinas (UNICAMP), Campinas, São Paulo, Brazil
- Steinberg et al. Intravesical sequential gemcitabine and docetaxel versus bacillus calmette-guerin (BCG) plus interferon in patients with recurrent non-muscle invasive bladder cancer following a single induction course of BCG. Urologic Oncology: Seminars and Original Investigations 40, 9.e1–9.e7 (2022).
- Packiam, V. T., Johnson, S. C. & Steinberg, G. D. Non-muscle-invasive bladder cancer: Intravesical treatments beyond Bacille Calmette-Guérin. Cancer 123, 390–400 (2017).
- Husseinzadeh, N. & Davenport, S. M. Role of toll-like receptors in cervical, endometrial and ovarian cancers: a review. Gynecol. Oncol. 135, 359–363 (2014).
- Galli, R. et al. TLR stimulation of prostate tumor cells induces chemokine-mediated recruitment of specific immune cell types. J. Immunol. 184, 6658–6669 (2010).
- W.J. Favaro, N.E.D. Caballero, Method for producing a nanostructured complex (CFI-1), a protein-associated nanostructured complex (MRB-CFI-1) and use, 11136242, 2021.
- Fávaro, W. J. et al. New synthetic nano-immunotherapy (OncoTherad®) for non-muscle invasive bladder cancer: Its synthesis, characterization and anticancer property. Tissue Cell 80, 101988 (2023).
- Website. https://ensaiosclinicos.gov.br/rg/RBR-6swqd2 (accessed March 12, 2023).
- Semple, J. W. & Freedman, J. Platelets and innate immunity. Cell. Mol. Life Sci. 67, 499–511 (2010).
- Morrissey, J. H., Choi, S. H. & Smith, S. A. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood 119, 5972–5979 (2012).
- Dias, L. P. et al. Effects of intravesical therapy with platelet-rich plasma (PRP) and Bacillus Calmette-Guérin (BCG) in non-muscle invasive bladder cancer. Tissue Cell 52, 17–27 (2018).
- Ribeiro de Souza, B. et al. A novel therapeutic strategy for non-muscle invasive bladder cancer: OncoTherad® immunotherapy associated with platelet-rich plasma. Int. Immunopharmacol. 123, 110723 (2023).
- Alonso, J. C. C. et al. Oncotherad immunotherapy elicits promising responses in Bacillus Calmette-Guérin-unresponsive non–muscle invasive bladder cancer: Results from phase I/ II study. J. Clin. Oncol. 38, e17048–e17048 (2020).
- Alonso, J. C. C. et al. Crosstalk among T-cell CX3CR1, immune checkpoints and toll-like receptor 4 signaling pathway in BCG-unresponsive nonmuscle invasive bladder cancer: Mechanism of action of OncoTherad Nano-Immunotherapy. J. Clin. Oncol. 40, e16551–e16551 (2022).
- OncoTherad May Prevent Tumor Recurrence in Non–Muscle-Invasive Bladder Cancer. Docwire News
- Fávaro, W. J. et al. Single-arm phase I/II study of the safety and efficacy of OncoTherad immunomodulator in patients BCG-refractory or relapsed non-muscle invasive bladder cancer. J. Clin. Oncol. 37, e16000–e16000 (2019).


