The role of these agents in the treatment of earlier stages of bladder cancer is an area of active investigation. Although cisplatin-based neoadjuvant chemotherapy (NAC) followed by radical cystectomy (RC) is considered the standard of care treatment for muscle invasive bladder cancer (MIBC), many of these patients are not able to receive this treatment due to other comorbidities impacting the tolerability of NAC or feasibility of RC. Some patients can be treated with radiation therapy (RT) but this treatment should be delivered concurrently with chemotherapy to improve its efficacy which again makes it difficult to use this treatment strategy in most cases that were not a good candidate for NAC and RC. Therefore, if proven to be effective, ICI and RT in combination with bladder preservation approaches might provide an alternative treatment for many MIBC patients.
DUART (BTCRC-GU15-023) is a phase II prospective study evaluating the safety, tolerability, and efficacy of concurrent durvalumab and RT followed by adjuvant durvalumab in patients with localized UC of the bladder with unresectable tumors, unfit for surgery, or unfit to receive cisplatin-based chemotherapy(1). Patients with T3-4 N0-2 M0 or T2 N1-2 M0 or any T N1-2 M0 were eligible to enroll in this study.
A total of 26 patients were enrolled in this study and were treated with durvalumab 1500 mg Q4 weeks for two doses along with definitive intent modulated RT (64.8Gy in 36 fractions over 7 weeks) followed by adjuvant durvalumab for one year. Overall, this treatment combination was well tolerated and no dose limiting toxicity or unexpected treatment related adverse events (AEs) were observed. The most common treatment related AEs were fatigue and diarrhea which were thought to be related to RT. Five patients discontinued treatment, four due to immune related AE (irAE). Radiation cystitis was reported in 6 patients (23%) grade 1-2. Progression free survival (PFS) probability at 1 year was 71.5% and the median PFS was 21.8 months. Also, 1-year OS probability was 83.8% with median overall survival (OS) of 30.8 months. Median PFS and OS were 25.1 and not reached in patients with node positive disease.

Figure – Clinical outcome [Journal for Immunotherapy of Cancer-Joshi et. al. 2023]1
Our correlative studies performed on pre- and post-treatment (if present) tumor samples, and peripheral blood resulted in thought provoking findings that require further validation in larger cohorts and future prospective studies. Although we observed a better PFS with high tumor mutational burden, no clinical correlation with baseline PD-L1 status was detected. Interestingly, flow cytometric analysis of peripheral blood samples showed high naïve CD4 T cells, low PD-1 positive CD4 T cells, and an increase in cytokine-producing CD8 T cells in blood samples from responders.
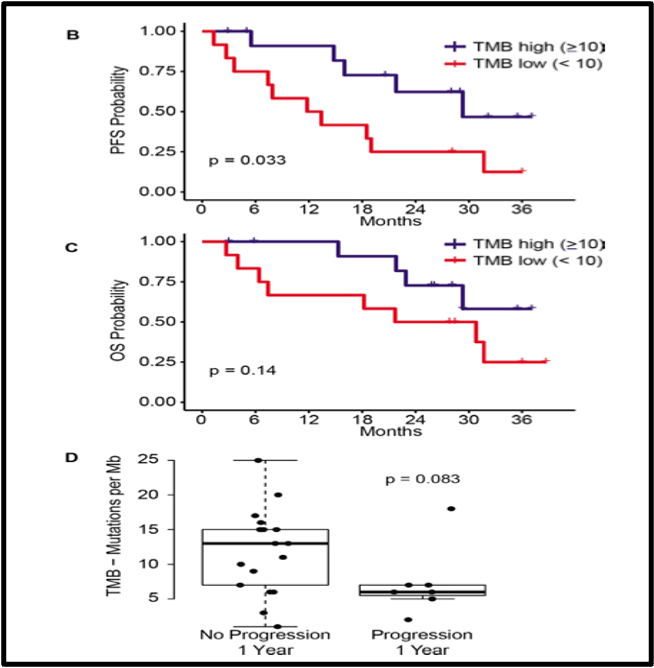
Figure- showing correlation between clinical outcome with high vs. low tumor mutational burden (TMB) [Journal for Immunotherapy of Cancer-Joshi et. al. 2023]1
Our study is among the first trials exploring the use of ICI and RT combination in patients with high risk localized MIBC including patients with nodal involvement. Our data show that a combination of durvalumab and RT can be a tolerable option with encouraging efficacy in this setting. In parallel with other ongoing trials, we hope to further investigate potential novel combinations and feasibility of bladder preserving approaches as an alternative option for patients with MIBC.
Written by: Hamid Emamekhoo, MD1 & Monika Joshi, MD, MRCP2
- Department of Medicine, University of Wisconsin-Madison Carbone Cancer Center, Madison, Wisconsin, USA
- Department of Medicine, Penn State Cancer Institute, Hershey, Pennsylvania, USA
Reference:
Read the Abstract


