We evaluated the retrospective data of 140 patients with metastatic urothelial carcinoma, registered to the Expanded-access program from thirty-five oncology centers in Turkey. One hundred and fifteen patients who received at least one cycle of atezolizumab treatment after progression with first-line chemotherapy were finally eligible for the study. The primary endpoint was the objective response rate (ORR). The secondary endpoints were overall survival (OS), progression-free survival (PFS), duration of response, and safety profile of patients. Kaplan Meier methods were used to calculate median follow-up and estimate PFS and OS.
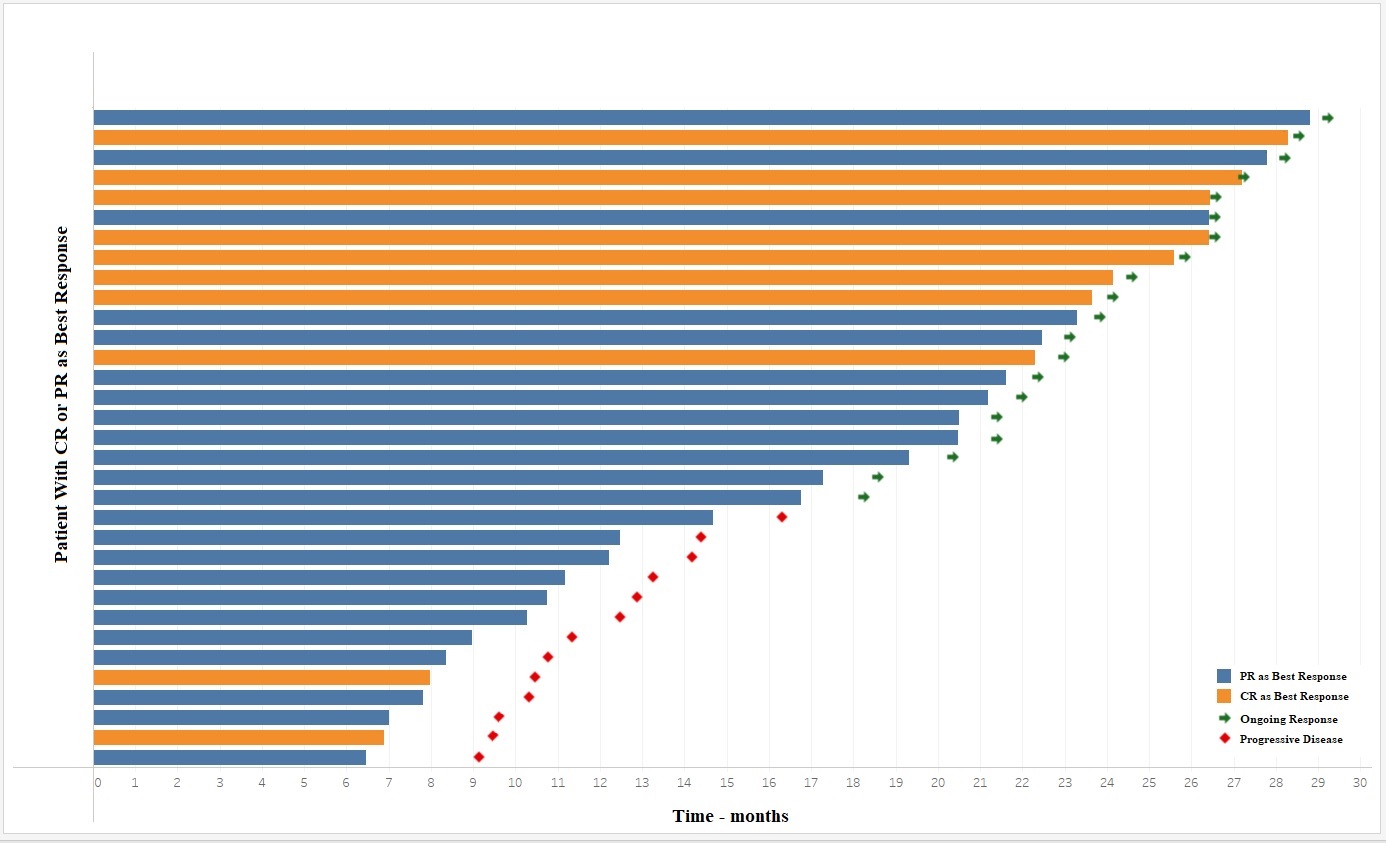
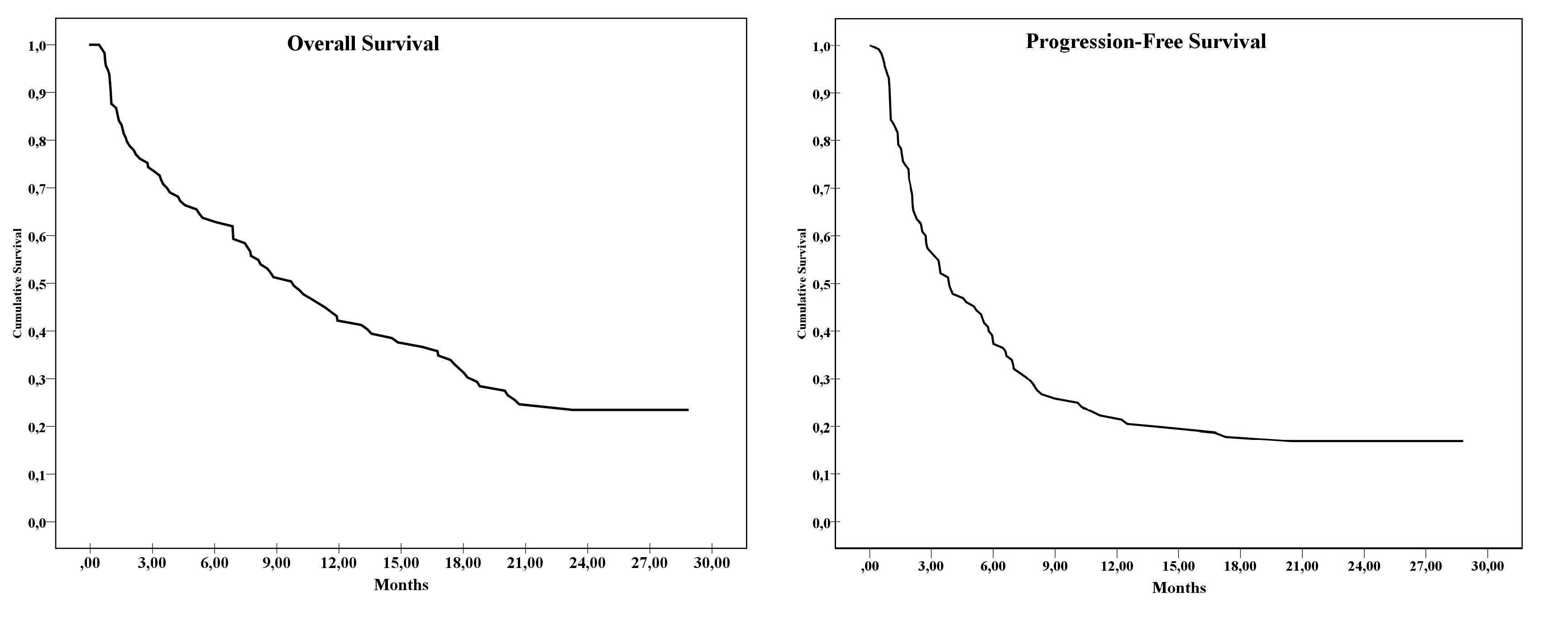
The median follow-up duration was 23.5 months. Complete response rate, partial response rate, and ORR were 8.7% (n = 10), 20.0% (n = 23), and 28.7% (n = 33), respectively. The median duration of response was 20.4 months (95% confidence interval [CI], 6.47–28.8). Of the 33 patients who responded to treatment, 60% (n = 20) had an ongoing response at the time of the analysis. PFS and OS with atezolizumab were 3.8 months (95% CI, 2.25–5.49) and 9.8 months (95% CI, 6.7–12.9), respectively.
All-cause and any grade adverse events were observed in 113 (98%) of patients. Sixty-four percent of patients experienced a treatment-related adverse event of any grade and 24 (21.2%) patients had a grade 3–4 treatment-related adverse event.
Limitations of the study included retrospective design and determination of treatment response based on clinical notes and local radiographic studies. We demonstrated in this retrospective study that atezolizumab is an effective and tolerable treatment for patients with urothelial cancer treated with atezolizumab after progression by the first-line chemotherapy. In our data, a broader range of patients was represented, compared with prior atezolizumab trials. Furthermore, there were a greater number of patients who previously received carboplatin as a first-line treatment for metastatic urothelial cancer and patients with ECOG PS 2 included in this study compared with the patients from Phase I-III atezolizumab trials. Thus, this study demonstrated that atezolizumab was also effective in a broad range of urothelial cancer patient populations after progression by the first-line chemotherapy.
Written by: Deniz Tural, Ömer Fatih Ölmez, Ahmet Taner Sümbül, Mehmet Artaç, Nail Özhan, Emre Akar, Burcu Çakar, Osman Köstek, Meltem Ekenel, Mustafa Erman, Hasan Şenol Coşkun, Fatih Selçukbiricik, Özge Keskin, Fatma Paksoy Türköz, Kerem Oruç, Selami Bayram, Uğur Yılmaz, İrem Bilgetekin, Birol Yıldız, Mehmet Ali Nahit Şendur, Nail Paksoy, Ahmet Dirican, Dilek Erdem, Meltem Selam, Özgür Tanrıverdi, Semra Paydaş, Zuhat Urakçı, Elif Atağ, Sabri Güncan, Yüksel Ürün, Ali Alkan, Ali Osman Kaya, Deniz Tataroğlu Özyükseler, Halil Taşkaynatan, Mustafa Yıldırım, Müge Sönmez, Tuğba Başoğlu, Şeyda Gündüz, Saadettin Kılıçkap
Bakirköy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey. Electronic address: ., Medipol University Hospital, Istanbul, Turkey., Medical Faculty, Baskent University, Adana, Turkey., Medical Faculty, Necmettin Erbakan University Meram, Konya, Turkey., Medical Faculty, Pamukkale University, Denizli, Turkey., Bakirköy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey., Medical Faculty, Ege University, Izmir, Turkey., Medical Faculty, Trakya University, Edirne, Turkey., Istanbul University Institute of Oncology, Istanbul, Turkey., Medical Faculty, Hacettepe University, Ankara, Turkey., Medical Faculty, Akdeniz University, Antalya, Turkey., Medical Faculty, Koc University, Istanbul, Turkey., Medical Faculty, Selcuk University, Konya, Turkey., MedicalPark Goztepe Hospital, Istanbul, Turkey., Medical Faculty, Istanbul University-Cerrahpasa, Istanbul, Turkey., Antalya Training and Research Hospital, Antalya, Turkey., MedicalPark Izmir Hospital, Izmir, Turkey., Dr. Abdurrahman Yurtaslan Ankara OncologyTraining and Research Hospital, Ankara, Turkey., Gulhane Training and Research Hospital, Ankara, Turkey., Ankara Yıldırım Beyazıt University, Faculty of Medicine, Ankara, Turkey., Medical Faculty, Celal Bayar University, Manisa, Turkey., MedicalPark Samsun Hospital, Samsun, Turkey., Liv Hospital, Istanbul, Turkey., Medical Faculty, Sitki Kocman University, Mugla, Turkey., Medical Faculty, Cukurova University, Adana, Turkey., Medical Faculty, Dicle University, Diyarbakir, Turkey., Medical Faculty, Dokuz Eylul University, Izmir, Turkey., Medical Faculty, Mersin University, Mersin, Turkey., Medical Faculty, Ankara University, Ankara, Turkey., Osmaniye State Hospital, Osmaniye, Turkey., Medicana Hospital, Istanbul, Turkey., Istanbul Kartal Dr. Lutfi Kirdar Training and Research Hospital, Istanbul, Turkey., Katip Celebi University Atatürk Training and Research Hospital, Izmir, Turkey., MedicalPark Gaziantep Hospital, Gaziantep, Turkey., Ordu State Hospital, Ordu, Turkey., Medical Faculty, Marmara University, Istanbul, Turkey., Antalya Memorial Hospital, Antalya, Turkey., Hacettepe University Institute of Oncology, Ankara, Turkey.
Read the Abstract


