While randomized controlled trials (RCTs) are gold standard in medical research [2, 3], RCTs are conducted in highly controlled environments and therefore their results may not carry over to the uncontrolled setting of real-life.[1] It is increasingly recognized that conclusions drawn from RCTs are not always a useful aid for decision-making because evaluating the value of a drug or technology requires an understanding of its impact on current clinical practice and management of patients in a real-life setting.[4]
Numerous studies have shown that testosterone therapy reduces waist circumference, body fat mass, blood pressure, blood glucose and HbA1c, increases lean body mass and insulin sensitivity, and improves lipid profiles.[5-13] Since obesity, dyslipidemia, insulin resistance, hyperglycemia, metabolic syndrome, hypertension and diabetes are well established cardiovascular risk factors, any therapeutic modality that improves these risk factors is expected to reduce cardiovascular risk. A series of “real-life studies” with an observation period of up to 17 years have confirmed the improvements in cardiovascular risk factors with long-term testosterone therapy, as well as its safety.[11, 13-30]
Despite this, a few flawed studies alleged that testosterone therapy would increase cardiovascular risk.[31-34] However, after having reviewed the available evidence on testosterone therapy and cardiovascular risk [35], the FDA denied the petition to place a black box warning about cardiovascular risk on testosterone products [36] and the European Medicines Agency (EMA) denied the allegations that testosterone therapy would increase cardiovascular risk.[37] Here we present the results from a new long-term study which confirms the safety of testosterone therapy and actually shows reduced cardiovascular risk and mortality.[14]
Of 656 men (mean age: 61 years) with total testosterone levels 12.1 nmol/L (349 ng/dL) or lower and symptoms of hypogonadism, 360 received testosterone undecanoate injections 1000 mg every 12 weeks following an initial 6-week interval, for up to 10 years. This treatment group was compared to a control group made up of 296 men who had opted to not receive testosterone therapy. Measurements were taken at least twice a year and 8-year data were analyzed.
Key points this study adds
- Long-term testosterone therapy for 8 years in obese men with testosterone deficiency reduces deaths and non-fatal heart attacks and strokes, compared to men not receiving testosterone therapy.
- The testosterone-group has an estimated reduction in mortality between 66% and 92% compared to non-treated men.
- As expected, hemoglobin and hematocrit increased in the testosterone treated group, but levels remained within the physiological reference ranges.
- In the testosterone treated group, 7 patients (1.9%) were diagnosed with low-grade prostate cancer. In the untreated (control) group, 12 patients (4.1%) were diagnosed with prostate cancer.
- This study confirmed the previously reported reductions in waist circumference, body weight and BMI. The improvements in lipid profile, especially a large reduction in non-HDL levels, and glycemic control as indicated by a marked reduction in HbA1c from diabetic to normal glycemia, are particularly notable.
- Being conducted in a real-life setting – as opposed to a controlled study environment – this study provides evidence that long-term testosterone therapy is both effective, safe and feasible in clinical practice, and importantly, results in a reduction in "hard clinical endpoints".
A significant improvement was seen in waist circumference, body weight, BMI, systolic and diastolic blood pressure, LDL, HDL, triglycerides and non-HDL, glycemic control and liver enzymes. Particularly notable are the marked reductions in non-HDL from 224 to 113 mg/dL (5.8 to 2.8 mmol/L) and HbA1c 6.9% to 5.6% in testosterone treated men, compared to increases in non-HDL and HbA1c from 194 to 201 mg/dL (5.0 to 5.2 mmol/L) and 6.1% to 6.4% respectively, in non-treated men.
Safety Parameters
As expected, hemoglobin and hematocrit increased in the testosterone treated group, but levels remained within the physiological reference ranges.
In the testosterone treated group 7 patients (1.9%) were diagnosed with low-grade prostate cancer. In the untreated (control) group 12 patients (4.1%) were diagnosed with prostate cancer.
Mortality and Nonfatal Myocardial Infarction (MI) and Stroke
There were 26 nonfatal MIs and 30 nonfatal strokes in the control group and none in the testosterone-treated group. There were 2 deaths in the testosterone-treated group, none was related to cardiovascular events. One was attributed to postsurgical thromboembolism and the other due to traffic accident. In the non-treated control group, there were 21 deaths, 19 of which were due to cardiovascular events (5 MI, 4 stroke, 7 heart failure, 2 thromboembolism, 1 lung embolism).
The estimated reduction in mortality for the testosterone-group was between 66% and 92%. Figure 1 illustrates the marked differences in mortality, MI and stroke incidences between the groups.
Figure 1: Differences in mortality, MI and stroke between testosterone treated men and non-treated men.
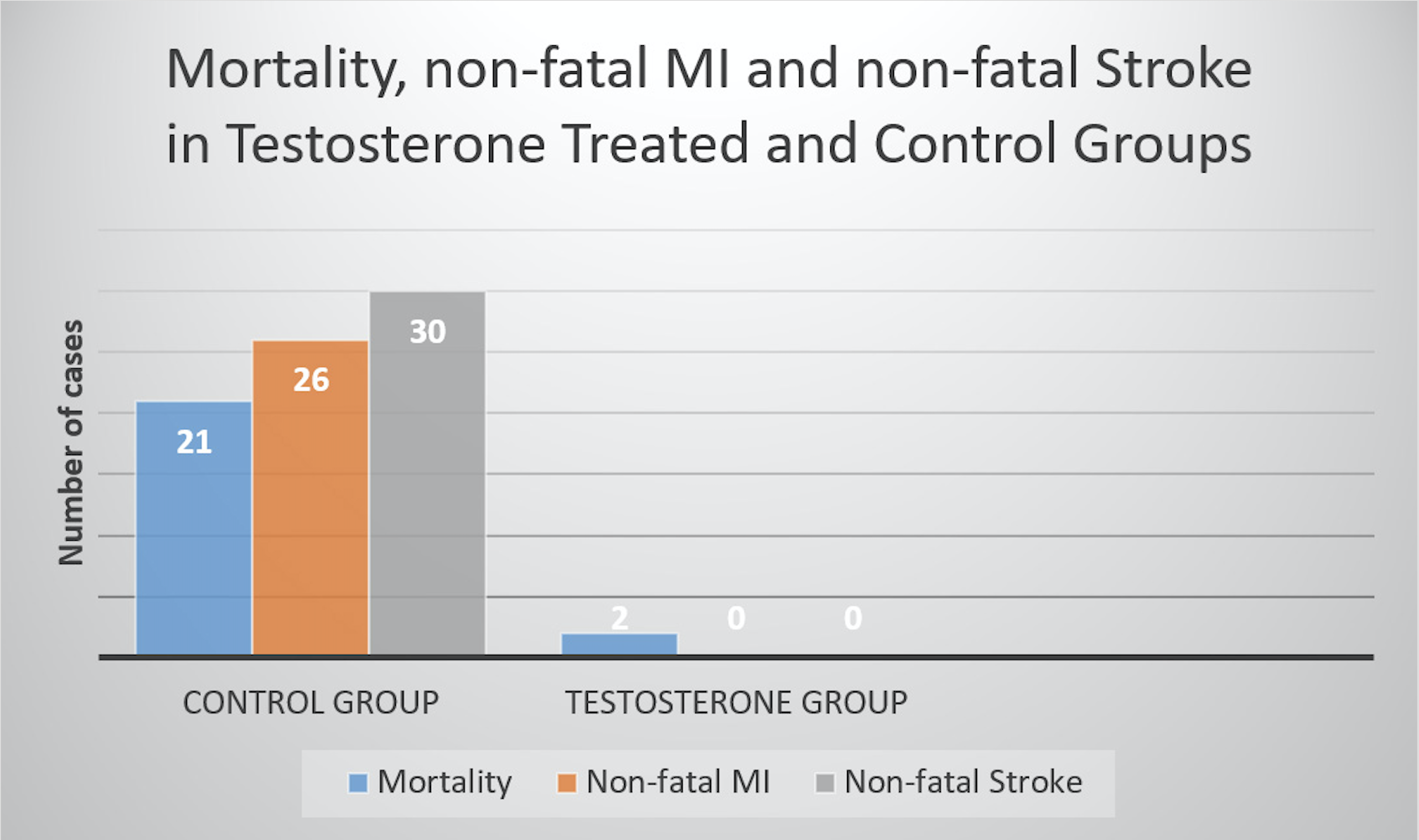
Data from Traish A, Haider A, Haider K, Doros G, Saad F. Long-Term Testosterone Therapy Improves Cardiometabolic Function and Reduces Risk of Cardiovascular Disease in Men with Hypogonadism: A Real-Life Observational Registry Study Setting Comparing Treated and Untreated (Control) Groups. Journal of cardiovascular pharmacology and therapeutics. 2017:epub
Comments
This is the first long-term observational study that includes a comparison (control) group.[14] It confirms the safety and efficacy found in previous long-term real-life studies [11, 13-30] and adds new important data. Several findings in this study deserve attention.
Glycemic control
HbA1c is used to diagnose prediabetes and diabetes. The threshold for diabetes is HbA1c ≥ 6.5% and prediabetes is present when HbA1c is between 5.7% and 6.49%.[44, 45] As seen in table 1, testosterone treatment reduced glycemia from the diabetic range (HbA1c 6.9%) to normal range (HbA1c 5.6%). Going from diabetic glycemia to normal glycemia is a marked improvement, as most interventions only reduce glycemia from prediabetic to normal or diabetic to prediabetic. As expected, in the control group there was a worsening in glycemic control with an increase in HbA1c from 6.1% to 6.4%, to near diabetic glycemia (HbA1c 6.5%).
non-HDL cholesterol
This study is also notable for reporting a marked reduction in non-HDL cholesterol (non-HDL or non-HDL-C).[14] When triglyceride levels exceed 150 mg/dL – as is commonly seen in patients with the metabolic syndrome, obesity, diabetes and cardiovascular disease - LDL particle number, apoB and VLDL levels increase without concomitant elevations in LDL-C.[54-56] Thus, non-HDL-C is more reflective of atherogenicity in people with elevated triglycerides.[57]
Several society guidelines for management of dyslipidemia for cardiovascular disease have recently added non-HDL as a primary treatment target. The International Atherosclerosis Society (IAS) Position Paper on the management of dyslipidemia considers non-HDL-C as an alternative to LDL-C as target of therapy, and actually favors adoption of non-HDL-C as the major target of lipid-lowering therapy.[57] The IAS expects that in future guidelines non-HDL-C will replace LDL-C as the best treatment target.
The European Society of Cardiology (ESC) / European Atherosclerosis Society (EAS) guideline states that non-HDL-C can provide a better risk estimation compared with LDL-C, in particular in patients with the metabolic syndrome or diabetes, who commonly have elevated triglyceride levels.[58]
Notably, the National Lipid Association (NLA) states that while non-HDL-C and LDL-C are co-primary treatment targets, non-HDL-C is the superior treatment target for modification.[59] Non-HDL-C levels and change during treatment of dyslipidemia are more strongly associated with reduced risk for atherosclerotic coronary heart disease (CHD) than changes in LDL-C, or on-treatment levels of LDL-C.[59] According to the 2017 AACE, ACE Dyslipidemia Clinical Practice Guidelines Update, the non-HDL-C treatment goal is 130 mg/dL for patients with two other risk factors, and 100 mg/dL for patients with two other risk factors plus diabetes or coronary, carotid or peripheral vascular disease.[60] The European Society of Cardiology (ESC) / European Atherosclerosis Society (EAS) guideline recommends non-HDL-C targets of 2.6, 3.3 and 3.8 mmol/L (100, 130 and 145 mg/dL) in patients with very high, high and low to moderate CV risk, respectively.[58] In light of this, the marked reduction in non-HDL from 5.8 to 2.8 mmol/L (224 to 113 mg/dL) in testosterone treated men, and increase from 5.0 to 5.2 mmol/L (194 to 201 mg/dL in non-treated men, should be highlighted.
Cardiovascular events and mortality
Regarding the debated issue of testosterone therapy and major cardiovascular events and mortality, it is of particular interest that there were only 2 deaths in the testosterone treated group and none was related to cardiovascular events. Notably, in the non-treated control group there were 21 deaths, 19 of which were related to cardiovascular events. Furthermore, there were 26 nonfatal MIs and 30 nonfatal strokes in the control group but none in the T-treated group. In addition to all the beneficial changes in waist circumference reduction, weight loss and metabolic parameters that many studies have shown before, this is the first long-term study with a control (non-testosterone treated) group showing that this actually translates into reductions of “hard clinical outcomes” in real-life.
The reduced incidence of MI, stroke, and mortality in testosterone treated hypogonadal men is in agreement with prior observational real-life studies [20, 61-71] and two large meta-analyses of RCTs.[72, 73] The safety and cardiovascular protection of testosterone therapy was confirmed in the more recent Testosterone Trials, which found that during the follow-up year after the first year of testosterone therapy, there were 8 heart attacks in the placebo group compared to 1 in the testosterone group.[74] The longest duration RCT conducted at this point lasted for 3 years and also confirmed the safety of testosterone therapy.[75] An interesting finding in this study – which was not reported in the abstract - was that in men not taking statins, one marker of atherosclerosis (coronary artery calcium) was significantly lower in the testosterone group than in the placebo group.[75]
It is also notable that another real-life study of men with testosterone deficiency and a history of cardiovascular disease, who received testosterone therapy for up to 8 years, no patient suffered a major adverse cardiovascular event during the full observation time.[20] In this study, cardio-metabolic parameters (lipid profile, glycemic control, blood pressure, heart rate, and pulse pressure), weight and waist circumference all improved significantly and sustainably [20], similarly to the results found in the study reported here.[14] Importantly, these improvements occurred even though patients were treated with statins, which suggests that statin treatment alone is inadequate. Thus, testosterone therapy may also be effective as an add-on treatment for secondary prevention of cardiovascular events in testosterone deficient men with a history of cardiovascular disease.[20]
Clinical practice relevance
Achievement of therapeutic testosterone levels is essential for benefits to occur.[66] The study reported here provides critical information on the long-term safety and effectiveness of testosterone therapy in clinical practice, as well as especially relevant data on adherence in the general population of men attending urological clinics.[14] It is notable that patients in the testosterone treated group achieved 100% medication adherence, as the testosterone injections were given in the doctor’s office and documented. This shows that injections of testosterone undecanoate at 12-week intervals is practically feasible in routine clinical practice, even in the long-term (the maximal observation duration was 10 years).[14]
The combined evidence base from both RCTs [72, 73] and observational studies [20, 61-70] is now strongly supportive of beneficial cardiovascular effects of testosterone therapy in hypogonadal men, which is accompanied by a reduction in cardiovascular mortality. In obese men, long-term testosterone therapy with testosterone undecanoate injections at 12 week intervals is clearly feasible in routine clinical practice and results in sustained weight loss and reductions in waist circumference and BMI without weight regain.[11, 13-30] Interestingly, the 8 year weight loss of 17-24% with testosterone therapy [14] compares favorably to the 10 year weight loss of 14-25% seen after bariatric surgery.[76] Considering that bariatric surgery is an invasive and expensive procedure that can only treat a minority of the rapidly growing obese population, non-surgical interventions that mimic the metabolic benefits of bariatric surgery, with a reduced morbidity and mortality burden, are tenable alternatives.[77] To put this in context, the Look AHEAD Trial – which investigated the effects of an intensive lifestyle intervention – found a weight loss 6 % in the intervention group (in the control group, the weight loss was 3.5%).[78] Obesity drugs (orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide) only result in a short-term (1 year) weight loss of around 5%.[79]
Hence, an accumulating body of evidence from long-term real-life studies of testosterone therapy in obese hypogonadal men suggests that testosterone therapy merits serious consideration among healthcare professionals who are dealing with the rapidly growing number of overweight/obesity patients.
Written By: Monica Caliber, MSc Nutrition, University of Stockholm & Karolinska Institute, Sweden Baylor University, TX, USA
References:
1. Cox, J.L. and K. Pieper, Harnessing the power of real-life data. European Heart Journal Supplements, 2015. 17(suppl_D): p. D9-D14.
2. Stanley, K., Design of randomized controlled trials. Circulation, 2007. 115(9): p. 1164-9.
3. Houle, S., An introduction to the fundamentals of randomized controlled trials in pharmacy research. Can J Hosp Pharm, 2015. 68(1): p. 28-32.
4. Annemans, L., M. Aristides, and M. Kubin. Real-Life Data: A Growing Need. The Official News & Technical Journal of The International Society for Pharmacoeconomics and Outcomes Research. Available at https://www.ispor.org/news/articles/oct07/rld.asp (accessed Febr 12, 2017).
5. Saad, F., Androgen therapy in men with testosterone deficiency: can testosterone reduce the risk of cardiovascular disease? Diabetes Metab Res Rev, 2012. 28 Suppl 2: p. 52-9.
6. Saad, F., et al., Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev, 2012. 8(2): p. 131-43.
7. Saad, F., et al., Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol, 2011. 165(5): p. 675-85.
8. Saad, F. and L.J. Gooren, The role of testosterone in the etiology and treatment of obesity, the metabolic syndrome, and diabetes mellitus type 2. J Obes, 2011. 2011.
9. Traish, A., Testosterone therapy in men with testosterone deficiency: Are we beyond the point of no return? Investig Clin Urol, 2016. 57(6): p. 384-400.
10. Saad, F., et al., Testosterone Deficiency and Testosterone Treatment in Older Men. Gerontology, 2017. 63(2): p. 144-156.
11. Francomano, D., A. Lenzi, and A. Aversa, Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol, 2014. 2014: p. 527470.
12. Traish, A.M., et al., Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract, 2014. 68(3): p. 314-29.
13. Yassin, A.A., et al., Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study. Andrologia, 2016: p. Jan 14. doi: 10.1111/and.12514. [Epub ahead of print].
14. Traish, A., et al., Long-Term Testosterone Therapy Improves Cardiometabolic Function and Reduces Risk of Cardiovascular Disease in Men with Hypogonadism: A Real-Life Observational Registry Study Setting Comparing Treated and Untreated (Control) Groups. Journal of Cardiovascular Pharmacology and Therapeutics, 2017: p. epub.
15. Saad, F., et al., Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond), 2016. 40(1): p. 162-70.
16. Yassin, A., et al., Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol (Oxf), 2016. 84(1): p. 107-14.
17. Yassin, A., et al., Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male, 2016. 19(1): p. 64-9.
18. Yassin, A.A., et al., Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study. Andrologia, 2016. 48(7): p. 793-9.
19. Saad, F., et al., Elderly men over 65 years of age with late-onset hypogonadism benefit as much from testosterone treatment as do younger men. Korean J Urol, 2015. 56(4): p. 310-7.
20. Haider, A., et al., Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag, 2016. 12: p. 251-61.
21. Francomano, D., et al., Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest, 2014. 37: p. 401-411.
22. Francomano, D., et al., Effects of 5-year treatment with testosterone undecanoate on lower urinary tract symptoms in obese men with hypogonadism and metabolic syndrome. Urology, 2014. 83(1): p. 167-73.
23. Haider, A., et al., Progressive Improvement of T-Scores in Men with Osteoporosis and Subnormal Serum Testosterone Levels upon Treatment with Testosterone over Six Years. Int J Endocrinol, 2014. 2014: p. 496948.
24. Haider, A., et al., Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: An observational study. Obes Res Clin Pract, 2014. 8(4): p. e339-49.
25. Haider, A., et al., Effects of long-term testosterone therapy on patients with "diabesity": results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol, 2014. 2014: p. 683515.
26. Haider, A., et al., Incidence of Prostate Cancer in Hypogonadal Men Receiving Testosterone Therapy: Observations from Five Year-median Follow-up of Three Registries. J Urol, 2015. 193(1): p. 80-86.
27. Saad, F., et al., Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring), 2013. 21(10): p. 1975-81.
28. Yassin, A. and G. Doros, Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes, 2013. 3(3-4): p. 73-83.
29. Yassin, D.J., et al., Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med, 2014. 11(6): p. 1567-76.
30. Permpongkosol, S., et al., Effects of 8-Year Treatment of Long-Acting Testosterone Undecanoate on Metabolic Parameters, Urinary Symptoms, Bone Mineral Density, and Sexual Function in Men With Late-Onset Hypogonadism. J Sex Med, 2016. 13(8): p. 1199-211.
31. Vigen, R., et al., Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA, 2013. 310(17): p. 1829-36.
32. Finkle, W.D., et al., Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One, 2014. 9(1): p. e85805.
33. Xu, L., et al., Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med, 2013. 11: p. 108.
34. Basaria, S., et al., Adverse events associated with testosterone administration. N Engl J Med, 2010. 363(2): p. 109-22.
35. Morgentaler, A., et al., Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc, 2015. 90(2): p. 224-51.
36. FDA Citizen Petition Denial Response from FDA CDER to Public Citizen, available at http://www.regulations.gov/#!documentDetail;D=FDA-2014-P-0258-0003, accessed Aug 31, 2014.
37. European Medicines Agency, PRAC review does not confirm increase in heart problems with testosterone medicines - Committee recommends medicines can continue to be given for their authorised uses. Avaliable at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/10/WC500175207.pdf Accessed Dec 14, 2015. 2014.
38. Petrick, A., et al., Utility of Ultrasound, Transaminases, and Visual Inspection to Assess Nonalcoholic Fatty Liver Disease in Bariatric Surgery Patients. Obes Surg, 2015. 25(12): p. 2368-75.
39. Haider, A., et al., Improvement of the metabolic syndrome and of non-alcoholic liver steatosis upon treatment of hypogonadal elderly men with parenteral testosterone undecanoate. Exp Clin Endocrinol Diabetes, 2010. 118(3): p. 167-71.
40. Hoyos, C.M., et al., Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol, 2012. 167(4): p. 531-41.
41. Kral, J.G., et al., Body fat topography as an independent predictor of fatty liver. Metabolism, 1993. 42(5): p. 548-51.
42. Mody, A., et al., Relevance of low testosterone to non-alcoholic fatty liver disease. Cardiovasc Endocrinol, 2015. 4(3): p. 83-89.
43. Lazo, M., et al., Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol, 2013. 178(1): p. 38-45.
44. American Diabetes Association, Standards of Medical Care in Diabetes-2017 Abridged for Primary Care Providers. Clin Diabetes, 2017. 35(1): p. 5-26.
45. American Diabetes Association, 2. Classification and Diagnosis of Diabetes. Available at http://care.diabetesjournals.org/content/40/Supplement_1 (accessed February 21, 2017). Diabetes Care, 2017. 40(Supplement 1): p. S11-S24.
46. Safar, M.E., B.I. Levy, and H. Struijker-Boudier, Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation, 2003. 107(22): p. 2864-9.
47. Hamilton, P.K., et al., Arterial stiffness: clinical relevance, measurement and treatment. Clin Sci (Lond), 2007. 113(4): p. 157-70.
48. Safar, M.E., Pulse pressure, arterial stiffness and wave reflections (augmentation index) as cardiovascular risk factors in hypertension. Ther Adv Cardiovasc Dis, 2008. 2(1): p. 13-24.
49. Zhao, L., et al., Brachial pulse pressure and cardiovascular or all-cause mortality in the general population: a meta-analysis of prospective observational studies. J Clin Hypertens (Greenwich), 2014. 16(9): p. 678-85.
50. Benetos, A., et al., Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension, 1998. 32(3): p. 560-4.
51. May, G.A. and F.J. Nagle, Changes in rate-pressure product with physical training of individuals with coronary artery disease. Phys Ther, 1984. 64(9): p. 1361-6.
52. Sembulingam, P., et al., Rate Pressure Product as a Determinant of Physical Fitness in Normal Young Adults. IOSR Journal of Dental and Medical Sciences, 2015. 14(4): p. 8-12.
53. Soundariya, K., V. Deepika, and S.P. Venkatesh, A comparative analysis of rate pressure product between prehypertensives and normotensives and its correlation with body mass index Indian Journal of Clinical Anatomy and Physiology, 2016. 3(4): p. 452-455.
54. Bays, H.E., et al., Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol, 2013. 7(4): p. 304-83.
55. Arsenault, B.J., S.M. Boekholdt, and J.J. Kastelein, Lipid parameters for measuring risk of cardiovascular disease. Nat Rev Cardiol, 2011. 8(4): p. 197-206.
56. Adiels, M., et al., Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol, 2008. 28(7): p. 1225-36.
57. Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel, An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia--full report. J Clin Lipidol, 2014. 8(1): p. 29-60.
58. Catapano, A.L., et al., ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis, 2011. 217(1): p. 3-46.
59. Bays, H.E., et al., National Lipid Association Annual Summary of Clinical Lipidology 2016. J Clin Lipidol, 2016. 10(1 Suppl): p. S1-S43.
60. Jellinger, P.S., et al., American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract, 2017.
61. Muraleedharan, V., et al., Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol, 2013. 169(6): p. 725-33.
62. Shores, M.M., et al., Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab, 2012. 97(6): p. 2050-8.
63. Baillargeon, J., et al., Risk of Myocardial Infarction in Older Men Receiving Testosterone Therapy. Ann Pharmacother, 2014. 48(9): p. 1138-1144.
64. Eisenberg, M.L., et al., Testosterone therapy and mortality risk. Int J Impot Res, 2015. 27(2): p. 46-8.
65. Tan, R.S., K.R. Cook, and W.G. Reilly, Myocardial Infarction and Stroke Risk in Young Healthy Men Treated with Injectable Testosterone. Int J Endocrinol, 2015. 2015: p. 970750.
66. Sharma, R., et al., Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J, 2015. 36(40): p. 2706-15.
67. Baillargeon, J., et al., Risk of Venous Thromboembolism in Men Receiving Testosterone Therapy. Mayo Clin Proc, 2015. 90(8): p. 1038-45.
68. Etminan, M., et al., Testosterone therapy and risk of myocardial infarction: a pharmacoepidemiologic study. Pharmacotherapy, 2015. 35(1): p. 72-8.
69. Anderson, J.L., et al., Impact of Testosterone Replacement Therapy on Myocardial Infarction, Stroke, and Death in Men With Low Testosterone Concentrations in an Integrated Health Care System. Am J Cardiol, 2016. 117(5): p. 794-9.
70. Ramasamy, R., et al., Association Between Testosterone Supplementation Therapy and Thrombotic Events in Elderly Men. Urology, 2015. 86(2): p. 283-5.
71. Cheetham, T., et al., Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Internal Medicine, 2017.
72. Corona, G., et al., Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf, 2014. 13(10): p. 1327-51.
73. Corona, G., E. Maseroli, and M. Maggi, Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother, 2014. 15(13): p. 1903-26.
74. Snyder, P.J., et al., Effects of Testosterone Treatment in Older Men. N Engl J Med, 2016. 374(7): p. 611-24.
75. Basaria, S., et al., Effects of Testosterone Administration for 3 Years on Subclinical Atherosclerosis Progression in Older Men With Low or Low-Normal Testosterone Levels: A Randomized Clinical Trial. JAMA, 2015. 314(6): p. 570-81.
76. Sjöström, L., et al., Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med, 2007. 357(8): p. 741-52.
77. Miras, A.D. and C.W. le Roux, Can medical therapy mimic the clinical efficacy or physiological effects of bariatric surgery? Int J Obes (Lond), 2014. 38(3): p. 325-33.
78. Look, A.R.G., et al., Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med, 2013. 369(2): p. 145-54.
79. Khera, R., et al., Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA, 2016. 315(22): p. 2424-34.
Read the Abstract


