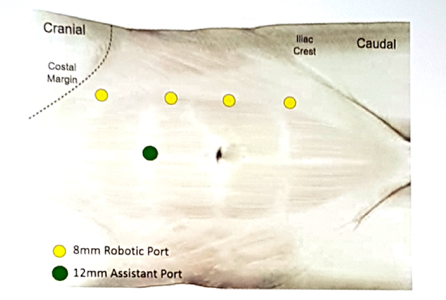
Dr. Ashok Hemal from Wake Forest Medical Center then presented his experience with robotic nephroureterectomy. Dr. Hemal started by noting that there are several ways to manage the distal ureter when performing a minimally invasive nephroureterectomy, including (i) stapled vs sutured closure of the bladder following resection, (ii) endoscopic detachment, which may include unroofing the ureter or the “pluck” technique, and (iii) ureteral intussusception. There have also been several modifications described to obstruct the ureter to prevent cell spillage, including clipping, fibrin glue, and the LigaSure. This management is crucial considering that recurrence of urothelial carcinoma in the residual sump has been reported in 13-75% of cases. According to Dr. Hemal, there are several modifications for port placement described in the literature by leaders in robotic surgery. This is how Dr. Hemal places his ports for robotic nephroureterectomy using the Xi system:
Dr. Hemal favors using indocyanine green (ICG) during robotic nephroureterectomy, specifically for intravesical instillation to aid in identification of the bladder cuff. Previously published work from his group on 65 patients undergoing robotic nephroureterectomy from 2008-2014 demonstrated pT2 in 65%, pT3 in 28.3%, and pT4 in 6.7% [1]. Over a median follow-up of 25.1 months (range 6 to 68.9), the 2 and 5-year overall survival was 86.9% and 62.6%, cancer specific survival was 92.9% and 69.5%, and recurrence-free survival was 65.3% and 57.1%, respectively. A total of 23 patients experienced disease recurrence. Bladder recurrence developed in 15 patients, 12 had isolated bladder recurrence and 8 had metastatic disease.
Dr. Hemal’s group has also compared their experience using the Si vs Xi da Vinci robotic systems. From April 2009 to December 2017, 87 patients underwent robotic nephroureterectomy, including 30 with the Xi and 57 with the Standard/S/Si [2]. Operative time using the Xi was shorter, 184 vs 232 minutes (p = 0.004), while other perioperative outcomes were similar between the two groups. More patients underwent lymph node dissection using the Xi system (p = 0.02), and complications were similar between groups. Interestingly, because of the shorter operative time, shorter anesthesia time, and ease of docking and working with the Xi, the cost for robotic nephroureterectomy was $1,250 USD less compared to the Standard/S/Si system. Dr. Hemal concluded his presentation stating that the robotic approach for nephroureterectomy allows you to adhere to sound oncological principles, including appropriate management of the kidney, lymph node dissection based on templates, distal ureteral management with a bladder cuff, and postoperative instillation of mitomycin.
Dr. Ronney Abaza concluded this session by discussing his experience with robotic radical nephrectomy. He started his presentation by highlighting the recently published JAMA article [3], which used the Premier Healthcare database from 2003 and 2015 to identify >23,000 laparoscopic and robotic nephrectomies, over which time period the use of robotic-assisted surgery increased substantially from 1.5% to 27%. In this study, the use of robotic-assistance was not associated with increased risk of any or major complications but was associated with prolonged operating time and higher hospital costs compared with laparoscopic surgery. Dr. Abaza’s rebuttal to this article is that during this time period there likely was some shift from open to robotic nephrectomy, increasing the minimally invasive pool in general and potentially explaining the longer operative times. In his opinion, this analysis is an unfair comparison without matching the cohorts. Dr. Abaza is an advocate for utilization of robotic nephrectomy for all-comers, regardless of size or complexity.
Recently, Dr. Abaza was part of a multi-institutional cohort reporting their experience with robotic nephrectomy and IVC thrombectomy [4]. A total of 32 cases were performed among nine surgeons at nine institutions and of these cases, 30 were level II and two were level III thrombi. Mean maximal tumor diameter was 9.6 cm (range 5.4-20), the length of inferior vena cava tumor thrombi ranged from 1 to 11 cm (median 4.2) on preoperative imaging. Mean operative time was 292 minutes (range 180-411) with a mean blood loss of 400 cc (range 25-2,000). There were no conversions to open surgery or aborted procedures and there were three transfusions of 1 to 3 units. There were seven patients who experienced distant recurrence at a mean follow-up of 15.4 months, including four who had node positive disease on postoperative pathological examination. Dr. Abaza’s conclusion is that even larger tumors (he’s performed several > 25 cm) and complex tumors (ie. with IVC thrombectomy) should be performed robotically in the hands of experienced robotic surgeons.
Presented By: Craig Rogers, Henry Ford, Vattikuti Urology Institute, Detroid, MI; Ashok Hemal, Wake Forest Baptist Medical Center, Winston-Salem, NC; Ronney Abaza, OhioHealth Dublin Methodist Hospital, Dublin, OH
Written By: Zachary Klaassen, MD, Urologic Oncology Fellow, University of Toronto, Princess Margaret Cancer Centre, @zklaassen_md ,at the 2018 North American Robotic Urology Symposium, February 16-17, 2018 - Las Vegas, NV
References:
1. Aboumohamed AA, Krane LS, Hemal AK. Oncologic outcomes following robotic-assisted laparoscopic nephroureterectomy with bladder cuff excision for upper tract urothelial carcinoma. J Urol 2015;194(6):1561-1566.
2. Patel MN, Hemal AK. Does advancing technology improve outcomes? Comparison of the da Vinci standard/S/Si to the Xi robotic platforms during robotic nephroureterectomy. J Endourol 2018;32(2):133-138.
3. Jeong IG, Khandwala YS, Kim JH, et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA 2017;318(16):1561-1568.
4. Abaza R, Shabsigh A, Castle E, et al. Multi-institutional experience with robotic nephrectomy with inferior vena cava tumor thrombectomy. J Urol 2016;195(4 Pt 1):865-871.


