Epidemiology
Upper tract disease represents approximately 5-10% of all urothelial malignancies.2 There is significant geographic variation in the incidence of these cancers, likely due to differences in the prevalence of underlying risk factors. In Balkan nations, UTUC may represent up to 40% of all kidney-related cancers. In Western nations, the incidence is approximately 2 per 100,000 population.3 As with bladder cancer, the incidence of these cancers peaks in individuals in their 8th and 9th decades of life.Interestingly, the incidence of UTUC appears to be increasing. Accompanying this has been a change in tumor stage, with an increasing proportion of earlier stage neoplasms.4
While bladder common is nearly 4 times as common in men as in women, the differential is closer to 2:1 for UTUC. Data is mixed on the association between patient gender and outcomes in UTUC. While some authors have reported that women are more likely to have advanced stage of disease5, other have not demonstrated this.6 Similarly, some groups report worse outcomes among women5 following nephroureterectomy, while other analyses have demonstrated no difference.6
Utilizing a large, multi-institutional cohort, Raman et al. examined predictors of recurrence and cancer-specific mortality among patients undergoing radical nephroureterectomy.7 They found that pathological tumor stage, nodal involvement, and tumor grade were associated with survival. Tumor location, whether in the renal pelvis or ureter, was not significantly associated with oncologic outcomes.
Etiology
Most, though not all, risk factors for the develop of UTUC are similar to the development of bladder cancer. Particular focus here is made of the unique risk factors including hereditary syndromes and uncommon environmental exposures.Genetic and environmental risk factors may contribute to the development of UTUC. Hereditary UTUC is associated with hereditary nonpolyposis colorectal carcinoma (HNPCC) syndrome, or Lynch syndrome.8 These patients may also have an increased risk of bladder cancers8, though whether this is from a urothelial field defect or seeding from the upper tract is unclear. HNPCC should be suspected among younger patients or those with a personal or family (two first-degree relatives) history of HNPCC-associated cancers, including colon or endometrial cancers.
A number of environmental risk factors are known for UTUC. First among these is aristolochic acid nephropathy. This is felt to be the common pathway between both Balkan endemic nephropathy and Chinese herb nephropathy (associated with consumption of Aristolochia fangchi) and UTUC.9 In Western countries, smoking is a much more common risk factor. This is associated with the production of N-hydroxylamine from aromatic amines. For reasons that are poorly understood, smoking seems to confer a higher risk of ureteral tumors than renal pelvic lesions.10 Previous reports suggested that coffee consumption may also be associated with UTUC but further work has suggested that these results likely represent Berkson's bias, due to the relationship between smoking and coffee consumption. Analgesic abuse, particularly of phenacetin, has also been well documented to be associated with the development of UTUC. However, the frequency of this exposure is rapidly declining and, as such, associated cases are relatively rare today. Arsenic exposure, typically through contaminated water, has also demonstrated an association with the development of UTUC. Interestingly, arsenic-associated UTUC demonstrates a female preponderance, unlike the general epidemiologic trends. As with bladder cancer, occupational exposures to aromatic hydrocarbons have been associated with a significantly increased risk of UTUC. Alkylating chemotherapy and chemic laxatives also appear to be associated with increased rates of UTUC. Finally, chronic inflammation may predispose to non-urothelial (squamous cell cancer or adenocarcinoma) of the upper urinary tract.
Association Between UTUC and Bladder Cancer
Significant focus has been directed to the relationship between UTUC and urothelial bladder cancer as a result of their shared tissue of origin. UTUC may occur in approximately 2 to 4% of patients with bladder cancer. However, there is wide variability in this quoted risk owing to differences in bladder cancer pathology and duration of follow-up. UTUC recurrence following bladder cancer treatment is reportedly more common among patients with carcinoma in situ (CIS) of the bladder11 and among those with more advanced (T1 vs Ta) disease.12Among patients with UTUC, recurrence in the bladder is relatively common. Depending on the report, estimates range from 15 to 75% at 5 years. Thus, routine cystoscopic surveillance is recommended following treatment for UTUC.
Histologic Considerations
The vast majority of upper tract tumors are urothelial in origin (>90%). As with bladder cancer, this may present as CIS, as papillary or sessile lesions, and as solitary lesions or in a multifocal pattern. Histological variants, now relatively well recognized in bladder cancer, may also be found in UTUC. Squamous cell cancers and adenocarcinomas make up a small proportion of upper tract malignancies. Other lesions including benign fibroepithelial polyps and neurofibromas as well as neuroendocrine tumors, hematopoietic tumors, and sarcomas have been reported.Two benign lesions, papillomas and inverted papillomas, have been associated with synchronous and metachronous development of UTUC. Thus, surveillance is recommended for patients with these lesions.
Clinical Assessment and Evaluation
The majority of patients with UTUC present with gross or microscopic hematuria. In fact, depending on estimates, up to 98% of all patients with UTUC will have hematuria. However, UTUC remains uncommon among patients presenting with hematuria. Â Flank pain may also occur and is typically felt to be due to obstruction of the collecting system. UTUC may also present entirely without symptoms as an incidental finding.Today, triphasic computed tomography (so called CT urography (CTU)) is the imaging modality of choice for the diagnosis of upper tract lesions. The sensitivity of CTU, as well as the negative predictive value, is reported to near 100%.13 Most upper tract lesions present with a filling defect. To distinguish from other causes of such a defect, UTUC typically have a density between 10 and 70 hounsfield units, less than radiolucent stones. In equivocal cases, retrography pyelography, selective ureteric washings for cytology, or ureteroscopy may be necessary.
Due to the association between UTUC and bladder cancer, cystoscopy is necessary to rule-out concomitant bladder cancer. Further, in the workup of a patient with hematuria, bladder cancer is a much more common underlying etiology than UTUC.
In addition to visualizing the lesion, ureteroscopy can allow for histologic diagnosis with biopsy or brushings. However, these biopsies are limited in the amount of tissue that may be samples and, as such, tumor grade is more reliable than stage based on these samples. Staging requires integration of imaging studies as tumor grade.
Cytology may be employed in the work-up of UTUC. While cytology is highly specific, it lacks sensitivity.
Staging, as detailed in the chart below, according to the TNM classification, parallels that of bladder cancer.
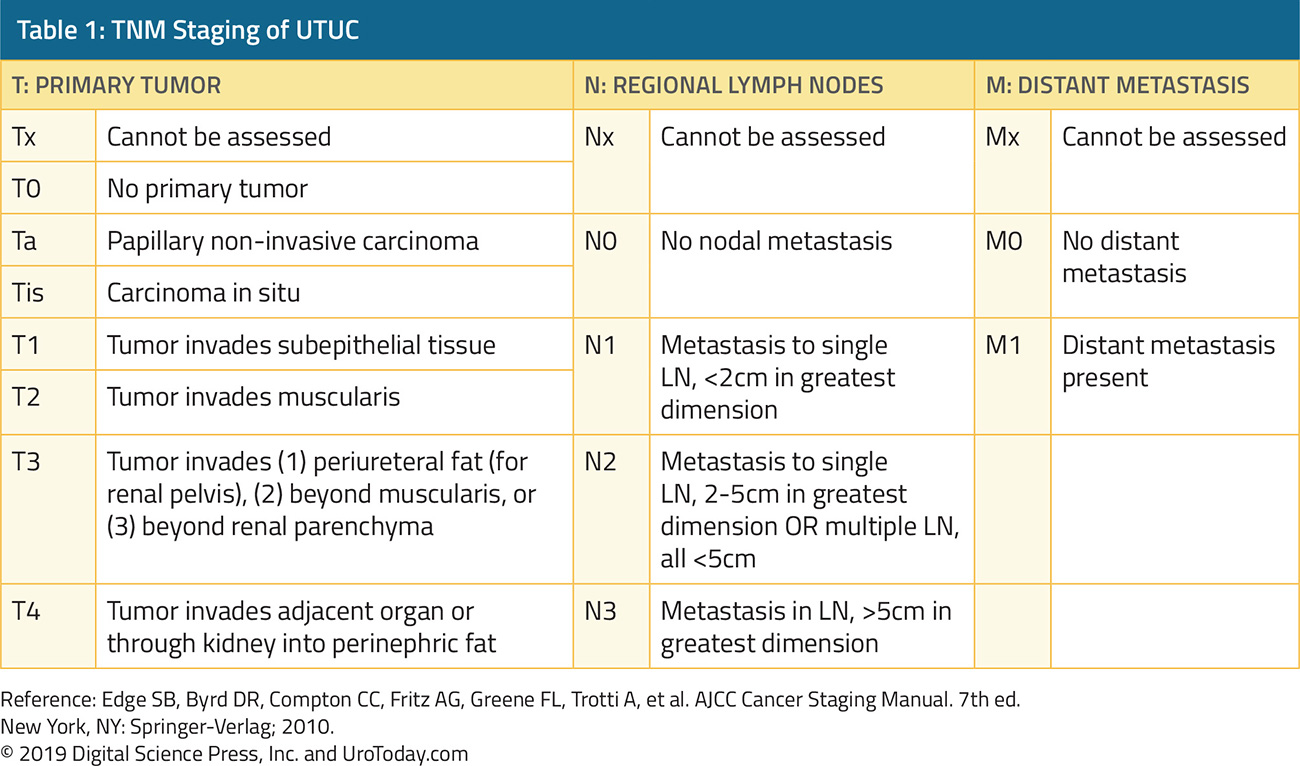
Upper tract tumors disseminate via lymphatic and hematogenous spread as well as direct extension. The most common sites of metastasis are lungs, liver, bones and lymph nodes. Thus, preoperative staging, depending on primary tumor characteristics, comprises thoracic imaging (CXR or CT), abdominal CT, liver function testing, and bone scan. In addition, for patients for whom nephroureterectomy is being considered, assessment of the contralateral renal function is necessary.
Prognostic Factors
Stage is the most important predictor. Unfortunately, it is sometimes difficult to ascertain stage preoperatively. Certainly, nodal involvement is independently associated with worse survival outcomes. Tumor location, whether in the renal pelvis or ureter, has proven controversial with regards to prognosis. While some studies have suggested no difference14, others have found improved survival among patients with renal pelvic tumors.15 Likely through its association with tumor stage, the presence of hydronephrosis has been shown to be associated with worse survival.16 Larger tumors (typically defined as greater than 3 or 4 cm) are also associated with worse outcomes. Other factors including tumor multifocality, tumor necrosis, and lymphovascular invasion have also been associated with worse outcomes though the data is somewhat contradictory.A number of molecular markers have been evaluated for prognostication in patients with UTUC. These include cytogentic abnormalities, oncogenes (c-MET and RON), as well as markers of apoptosis (Bcl-2 and surviving), markers of cell migration and invasion (E-cadherin and MMPs), cell cycle progression (p53 and CDKN1B), angiogenesis (HIF-1α), cell proliferation (Ki-67, EGFR, and NF-κB), cell differentiation (uroplakin III and snail), mitosis (aurora-A), and microsatellite instability.
Treatment
The relative rarity of UTUC has precluded many large trials to guide treatment for these patients. As an overarching principle, the least invasive treatment necessary for safe oncologic control of the tumor should be preferred. Depending on tumor characteristics, this may include radical nephroureterectomy (whether open or laparoscopic), segmental ureterectomy, and endoscopic/percutaneous tumor ablations.Radical nephroureterectomy remains the gold standard for large, high-grade and suspected invasive tumors of the renal pelvis and proximal ureter. A variety of techniques exist for management of the distal ureter though formal excision of a bladder cuff is the gold standard approach.
For patients with low-grade, non-invasive tumors, retrograde endoscopic or percutaneous ablation offer the potential for nephron-sparing treatment.
Perhaps the most notable advance in the treatment of patients with UTUC comes with the recent publication of the POUT trial which assessed the role of adjuvant chemotherapy following nephroureterectomy. Among 248 patients with pT2-4 N0-3 UTUC, Birtle and colleagues randomized patients to 4 cycles of adjuvant gemcitabine-cisplatin or surveillance. They demonstrated a significant improvement in disease-free survival and progression-free survival.
The multifocal and recurrence nature of urothelial carcinoma makes ongoing follow-up critical following any treatment. For patients opting for endoscopic approaches, repeated surveillance ureteroscopy is required. For other patients, cystoscopy, urine cytology and upper tract imaging are required. For patients at increased risk of metastases, thoracic imaging, biochemical studies including liver function testing, and bone scan may be indicated.
Published Date: January 28th, 2019


