Bladder cancer presents an ever increasing health care burden across the globe. The large majority of patients diagnosed with bladder cancer are over the age of 55, with an average age at the time of diagnosis of 73 and an increasing percentage 80 years and older.1 Men are about three to four times more likely to get bladder cancer during their lifetime than women.1
Although the causes of bladder cancer are unknown; the risk factors associated include cumulative cigarette smoke exposure (the most important risk factor).2 Importantly, the risk factors for bladder cancer are also associated with cardiac disease and diseases leading to decreased pulmonary function. Thus, most bladder cancer patients are afflicted with several comorbidities, adding to the complexity of management in patients with bladder cancer.
Radical cystectomy with neoadjuvant chemotherapy is the gold standard treatment for muscle invasive urothelial bladder tumors.3 While neoadjuvant chemotherapy is one of the few pieces of level one evidence we have for the treatment of bladder cancer, this remains vastly underutilized.3 Despite the robust data supporting neoadjuvant chemotherapy use, only approximately one-third of patients receive neoadjuvant chemotherapy. The reluctance to administer neoadjuvant chemotherapy is based on perception of increased morbidity with age, delay in surgery, and the opinion that this might add to complications of surgery. 4 However, there is no data to support this perception and to the contrary, in studies there has been no difference in complications or readmissions based on neoadjuvant chemotherapy status.5,6
Radical cystectomy accounts for approximately 10,000 cases annually. It is among the most complex urologic operations, and associated with considerable morbidity and prolonged inpatient stay. The single most reported quality indicator for radical cystectomy is length of stay, which remains high in the 8-9 day range in the United States and 15-20 days in Europe.7 It is absolutely true that radical cystectomy with bilateral pelvic lymph node dissection and urinary diversion is associated with a high post-operative morbidity of between 30% and 64%, even in high-volume centers.8 Of these complications, 15% are high-grade and there is an approximate 25-30% hospital readmission rate.9,10 Thus, we need to optimize patients prior to surgery, not only to improve on post-surgical outcomes, but also to potentially facilitate higher utilization of appropriate neoadjuvant chemotherapy.
In the ‘classic,’ ‘traditional care’ or pre-enhanced recovery after surgery (ERAS) pathway, a typical course for a patient undergoing radical cystectomy was associated with interventions that sometimes increase the patient’s physical and emotional stress. In addition, some previous interventions were based on surgical or anesthesia dogma rather than high quality trials. One of the most distressing elements to patients was a harsh bowel preparation (bowel prep) which included both mechanical and antibiotic treatment in combination with starvation beginning with a clear liquid only diet up to 2 days prior to surgery and nothing to eat or drink after the midnight before surgery. The practices of bowel prep and starvation were aimed at decreasing surgical site infections, however, this often lead to the unintended consequences of early hospital admissions, dehydration, sleep disruption and unneeded burdensome physical and emotional perioperative stress. Aggressive hydration was often needed to balance the bowel preparation, sometimes patients were receiving an excess of 10 liters of fluid intraoperatively. Postoperatively the patient was kept with a nasogastric tube and was permitted nothing by mouth until flatus, which could be 3 to 4 days of starvation after surgery. The final parameter in which pre-ERAS patients were managed includes copious opioid administration for pain control, often leading to a decrease in mental acuity and mobility as well as the unintended gastrointestinal (GI) side effects of nausea, vomiting, ileus and constipation. In the United States, the typical length of a hospital stay with traditional care is has been as long as 10 days which may include some time spent in the Intensive Care Unit or an extended recovery stay.11
What is Enhanced Recovery after Surgery (ERAS)?
Patients and providers are often not understanding of what their surgical journey will truly represent. A patient undergoing radical cystectomy easily can exceed the limits of their bodies to endure the physical and emotional toll of a 6 hour to 8 hour surgery and the recovery there after. In a frail patient with multiple comorbidities, this often leads to rapid deconditioning and suboptimal outcomes. ERAS pathways were created to reduce the physical and emotional stress for patients undergoing major surgical interventions.
ERAS pathways were first introduced in the 1990s as a means to reduce morbidity and improve effectiveness of care; the major thrust was in Europe where colorectal surgeons led the charge. Since then this has been adopted stateside and several meta-analyses have shown accelerated recovery, decreased hospital stay and improved quality of life for patients with ERAS in colorectal surgery. Thus, the use of ERAS is now routinely encouraged after colorectal and other major GI procedures.12,13 One meta-analysis revealed a consistent improvement in the quality of care by enabling standardization of health care processes using ERAS.14 Moreover, while accelerating recovery and safely reducing hospital stay, ERAS pathways optimize utilization of health care resources. The ERAS nature requires a multimodal approach focused on peri-operative period. Meta-analyses dependably find improved patient recovery after surgery, improved physiologic and psychological responses, and discharge to home earlier, all without compromising safety and wellbeing. Based on the favorable outcomes with ERAS for colorectal cancer, this paradigm has been adopted by high volume centers for bladder cancer surgery with resulting improvements in care for our patients.8
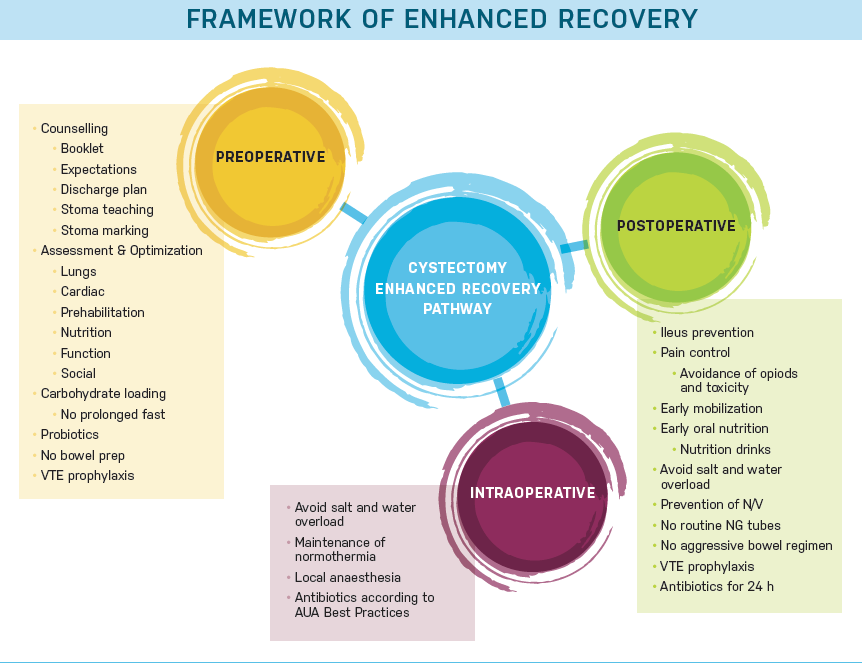
ERAS works best within the perioperative spectrum comprised of pre-, intra- and post-operative parameters (Figure 1).15 In the preoperative environment, expectations are set and optimal care for medical co-morbidities is undertaken. Optimization of nutrition is important and includeds discussion of pre- and post-operative recommended intake. Short term dietary modification to increase protein and carbohydrates in their diet and advice to decrease alcohol intake may be helpful. Smoking cessation is encouraged and cessation therapy may be recommended. Prehabilitation is also recomended, this can be something as simple as walking 30 minutes twice daily. While true prehabilitation training program with physical therapy, physiatry or geriatrics would be ideal, due to the time sensitive nature of bladder cancer, we often cannot delay surgery more than 4-6 weeks. Even though this is a short time span, it must be remembered and emphasized to the patient and family that any physical conditioning, even for one week, is helpful. Intesive bowel preparation is avoided. The patient should be given written and oral pre-admission information describing the specific aspects of the process: what will happen during hospitalization, what they should expect with respect to length of stay and whether there will be a home plan for a visiting nurse or home health aide, who will be at home with the patient after discharge. Most importantly, the patient and his or her support system should actively be engaged and play a role in their preparation and recovery process.15
Efficient and expeditious surgery is of utmost importance. Today surgeons use a smaller incision, and less bowel manipulation. Additional goals of surgery are to minimizie blood loss and the need for transfusion. In the ERAS intraoperative framework opioids are avoided and non-narcotic analgesia is provided intraoperatively. Intraoperative goal directed fluid therapy also plays a role in decreasing postoperative morbidity.8 Local anesthetic should be used at the incision sites. Multiple modes of local pain blockade can be used alone or in combination, these include: long acting local anesthetic (e.g. Exparel ®) in the incision, On-Q® pumps with continuous local anesthetic infusion, epidurals, quadratus lumborum blocks or transversus abdominal (TAP) blocks.8 The use of local anesthetics also allows for early mobilization, which is essential to the ERAS pathway. Patients are encouraged to be out of bed the night of surgery and ambulate at least four times daily all of which cannot be done if the patient has intense pain.
Avoidance of opiods is continued into the postoeperative period to reduce ileus and encourage mobilization. Prior to ERAS implementation, postoperative ileus, or lack of GI motility, was the most common cause for prolonged hospitalization and readmission, occurring in 12-25% after radical cystectomy.16 Lack of GI recovery contributes to morbidity and increased risk of secondary clinical consequences, such as nosocomial infections and adding a substantial cost burden to the healthcare system.17-19 Pharmacological prophylaxis is recommended to avoid postoperative nausea or vomiting, and early enteral feeding is encouraged. Nasogastric tubes are to be avoided. Alvimopan is administered as one dose preoperatively (before opioid exposure) and then twice daily usually until discharge to home. Alvimopan is a peripherally acting μ-opioid antagonist, with limited ability to cross the blood–brain barrier, and indicated for the prevention of postoperative ileus.20 It was approved for use in radical cystectomy based on the pivotal phase 4, randomized double-blind, placebo controlled clinical study of 280 patients.21 The results of this study showed a significant decrease compared to placebo in overall postoperative ileus related morbidity, nasogastric tube use, and length of hospital stay with no adverse safety concerns or increases in readmissions.21
Successful implementation of ERAS
The use of ERAS after radical cystectomy has been demonstrated to reduce hospital stay in several studies without increasing complications or readmissions.15,22,23 Studies using matched and unmatched historical comparisons and randomization to compare ERAS to traditional care have reported many positive outcomes in addition to length of stay reduction. An important factor is also the added improvement in quality of life for the first three days after surgery in ERAS patients compared to traditional care patients.23 Additional positive ERAS improvements in various studies have been decreased post-operative wound healing disorders, GI complications, fevers and thromboses.15,22,23 Interestingly, analgesic demand has also been significantly lower ERAS groups and patients eat more food in relation to the amount of food they are offered.15,23
Reduced cost and readmissions
ERAS protocols for radical cystectomy aim to improve patient care, reduce complications, and shorten hospital stay while potentially reducing health care expenditures. In one study of 201 consecutive patients at a single institution, half were managed with ERAS and the remaining half were managed with traditional management.24 In this study, improved clinical benefits and cost savings were seen in the ERAS arm when compared to the traditional management.24 The cost center–specific expenditures of the ERAS protocol for radical cystectomy, demonstrated a $4489 savings in 30-day costs relative to standard management.24 Despite a reduction in some complications and costs with ERAS, readmission rates for cystectomy have remained stable over the past decade. Radical cystectomy has one of the highest readmission rates of any surgery, at about 30%.9,10
Analysis of government and hospital databases reveal readmission rates for cystectomy exceed rates of esophagectomy, lung resection, and pancreaticoduodenectomy (Whipple), and exceed all other urologic surgeries.25 Overall, 30-day and 90-day readmission rates ranged from 13% and 23% for lung resection and 30% and 43% for cystectomy, respectively.25 In 2012 the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database identified patients undergoing radical prostatectomy, radical or partial nephrectomy and radical cystectomy who had unplanned readmission within 30 days after surgery, and the causes of their readmission.26 Radical cystectomy, again, had the highest burden of readmission compared with the other urological surgeries assessed in the study.26 Most readmissions occur early, within the first week after surgery and 77% are readmitted within 2 weeks of discharge.27 These patients tend to stay in the hospital for 7 days or more when they experience a readmission.27
Unfortunately despite adherence to most recent perioperative antibiotic guidelines, the incidence of readmissions after radical cystectomy due to infection still remains significant.28 Reasons for readmission change over time was shown in a population based study of 1782 patients.29 During the 6-year study period, 25.5% of patients undergoing cystectomy were readmitted within 30 days of discharge.29 Depending on the time from discharge the reasons for readmission varied. Infectious diagnoses were most prevalent by 30 days after discharge whereas GI and failure to thrive diagnoses appeared more common in the first 5 days after discharge.29 The most common new diagnoses at readmission involved infection, failure to thrive, and urinary and GI etiologies.29 Going forward, close surveillance of how imminent policy reforms affect patterns and quality of care will be necessary.
For patients undergoing cystectomy, substantial increases in the use of post-acute care yield reductions in hospitalization.The post discharge time period focuses on the first few weeks after discharge with emphasis on modifiable readmission factors. Several reviews and meta-analyses document that early follow-up may reduce readmissions by 20%.30 Follow-up care should include vigilance in looking for complications, a complication caught early may avoid a readmission. Communication between the patient and the physician is extremely important in the postoperative period. There should be early follow up with the patient’s primary care provider, in addition to early and frequent phone calls from their surgeon.31,32 With technology as a fact of life, tele-medicine and use of mobile apps are helpful.33,34 The most common categories of interventions which have been studied and reduced readmissions include patient education, discharge planning, follow-up telephone calls, patient-centered discharge instructions, and discharge coaches or nurses who consistently interact with the patient before and after discharge.30
In summary implementation of the ERAS pathway begins before surgery, when expectations are communicated and discussed prior to the actual admission. At this time the patient and physician identify and treat comorbidities emphasizing physical conditioning and proper nutrition. Reductions in ileus are expected from ERAS principles including: bowel preparation avoidance, decrease bowel handling intraoperatively, use of alvimopan, avoidance of opioids, and use of local analgesias. Expeditious surgery helps to minimize time under anesthesia with the additional goal of minimizing blood loss. The patient should be an active participant in his or her recovery as soon as possible with simple measures such as early mobilization. Oral intake is begun early, with rapid advancement from liquid to regular diet. Of couse, medical judgement should be exercised here, especially in older patient at risk of aspiration. Beginning early oral intake helps with caloric needs and a improves the patients sense of wellbeing. At discharge it is imperative that the patient should be ready both mentally and physically to take care of themselves with minimal assistance and have support at home from family and friends. When discussing expectations it is important to remember that they may vary among cultures and sensitivity is required. Once a patient is discharged, care continues and the first two weeks after discharge are critical for followup and readmission prevention.
ERAS as a multidisciplinary approach involving surgeons, anesthesiologists, nurses, dieticians, and allied health professionals is one of the key paradigm shifts that is now needed to optimize recovery of our patients undergoing radical cystectomy. No single intervention significantly reduces morbidity, but the combination of many interventions at all levels of the pathway is likely to accelerate the patient journey from diagnosis to return of normal function. The traditional pathway after diagnosis of muscle invasive bladder cancer has, unfortunately, not actively involved patients in the process (other than decision making). It is imperative that we include the patient – and their family/loved ones – in the entire process, from the time of diagnosis to the time of complete recovery and transition to survivor status.
Written By: ASHISH M KAMAT, MD, MBBS, FACS, is a Professor (Tenure) of Urology and Director of Urologic Oncology Fellowship at M.D. Anderson Cancer
Center, and a graduate of the AUA Leadership Program. Dr Kamat has authored over 200 publications, editorials & book chapters in prestigious journals; he is listed in ‘Who’s Who in Medicine’ and ‘Best Doctors in America’ and has won the Compassionate Doctor Award from patient groups. He is an exceptional educator nominated twice for the Robert M. Chamberlain Distinguished Mentor Award and has been invited as a visiting professor to several universities across the world. Dr Kamat is Co President, International Bladder Cancer Network, Chair, Bladder Cancer Think Tank (2015), Chair, Bladder Cancer Task Force for SITC,
JANET BAACK KUKREJA, MD, MPH, is a fellow in Urologic Oncology at the University of Texas MD Anderson Cancer Center. She completed her residency at the University of Rochester. In addition she received her MPH from the University of Rochester School of Medicine and Dentistry. Dr. Kukreja is invested in efforts to improve care and quality of urology patients through all areas of health services research.
References:
1. Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96-108.
2. Wilcox AN, Silverman DT, Friesen MC, et al. Smoking status, usual adult occupation, and risk of recurrent urothelial bladder carcinoma: data from The Cancer Genome Atlas (TCGA) Project. Cancer Causes Control. 2016;27(12):1429-1435.
3. Clark PE, Spiess PE, Agarwal N, et al. NCCN Guidelines Insights: Bladder Cancer, Version 2.2016. J Natl Comp Canc Netw. 2016;14(10):1213-1224.
4. Cowan NG, Chen Y, Downs TM, et al. Neoadjuvant chemotherapy use in bladder cancer: a survey of current practice and opinions. Adv Urol. 2014;2014:746298.
5. Johnson DC, Nielsen ME, Matthews J, et al. Neoadjuvant chemotherapy for bladder cancer does not increase risk of perioperative morbidity. BJU Int. 2014;114(2):221-228.
6. Gandaglia G, Popa I, Abdollah F, et al. The effect of neoadjuvant chemotherapy on perioperative outcomes in patients who have bladder cancer treated with radical cystectomy: a population-based study. Eur Urol. 2014;66(3):561-568.
7. Databases H. Healthcare Cost and Utilization Project (HCUP. 2017; www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed December 5, 2016.
8. Patel HR, Cerantola Y, Valerio M, et al. Enhanced recovery after surgery: are we ready, and can we afford not to implement these pathways for patients undergoing radical cystectomy? Eur Urol. 2014;65(2):263-266.
9. Jacobs BL, Zhang Y, Tan HJ, et al. Hospitalization trends after prostate and bladder surgery: implications of potential payment reforms. J Urol. 2013;189(1):59-65.
10. Gore JL, Lai J, Gilbert SM; Urologic Diseases in America Project. Readmissions in the postoperative period following urinary diversion. World J Urol. 2011;29(1):79-84.
11. Kumar AS, Kelleher DC, Sigle GW. Bowel preparation before elective surgery. Clin Colon Rectal Surg. 2013;26(3):146-152.
12. Adamina M, Kehlet H, Tomlinson GA, et al. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149(6):830-840.
13. Eskicioglu C, Forbes SS, Aarts MA, et al. Enhanced recovery after surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg. 2009;13(12):2321-2329.
14. Grant MC, Yang D, Wu CL, et al. Impact of enhanced recovery after surgery and fast track surgery pathways on healthcare-associated infections: results from a systematic review and meta-analysis. Ann Surg. 2017;265(1):68-79.
15. Baack Kukreja JE, Kiernan M, Schempp B, et al. Quality improvement in cystectomy care with enhanced recovery (QUICCER) study. BJU Int. 2017;119(1):38-49.
16. Chang SS, Baumgartner RG, Wells N, et al. Causes of increased hospital stay after radical cystectomy in a clinical pathway setting. J Urol. 2002;167(1):208-211.
17. Asgeirsson T, El-Badawi KI, Mahmood A, et al. Postoperative ileus: it costs more than you expect. J Am Coll Surg. 2010;210(2):228-231.
18. Doorly MG, Senagore AJ. Pathogenesis and clinical and economic consequences of postoperative ileus. Surg Clin North Am. 2012;92(2):259-272, viii.
19. Ramirez JA, McIntosh AG, Strehlow R, et al. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: a systematic review. Eur Urol. 2013;64(4):588-597.
20. Kraft M, Maclaren R, Du W, Owens G. Alvimopan (entereg) for the management of postoperative ileus in patients undergoing bowel resection. P T. 2010;35(1):44-49.
21. Lee CT, Chang SS, Kamat AM, et al. Alvimopan accelerates gastrointestinal recovery after radical cystectomy: a multicenter randomized placebo-controlled trial. Eur Urol. 2014;66(2):265-272.
22. Daneshmand S, Ahmadi H, Schuckman AK, et al. Enhanced recovery protocol after radical cystectomy for bladder cancer. J Urol. 2014;192(1):50-55.
23. Karl A, Buchner A, Becker A, et al. A new concept for early recovery after surgery for patients undergoing radical cystectomy for bladder cancer: results of a prospective randomized study. J Urol. 2014;191(2):335-340.
24. Nabhani J, Ahmadi H. Schuckman AK, et al. Cost analysis of the enhanced recovery after surgery protocol in patients undergoing radical cystectomy for bladder cancer. Eur Urol Focus. 2016;2:92-96.
25. Stitzenberg KB, Chang Y, Smith AB, Nielsen ME. Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol. 2015;33(5):455-464.
26. Schmid M, Chiang HA, Sood A, et al. Causes of hospital readmissions after urologic cancer surgery. Urol Oncol. 2016;34(5):236.e1-e11.
27. Skolarus TA, Jacobs BL, Schroeck FR, et al. Understanding hospital readmission intensity after radical cystectomy. J Urol. 2015;193(5):1500-1506.
28. Altobelli E, Buscarini M, Gill HS, Skinner EC. Readmission rate and causes at 90-day after radical cystectomy in patients on early recovery after surgery protocol. Bladder Cancer. 2017;3(1):51-56.
29. Hu M, Jacobs BL, Montgomery JS, et al. Sharpening the focus on causes and timing of readmission after radical cystectomy for bladder cancer. Cancer. 2014;120(9):1409-1416.
30. Kripalani S, Theobald CN, Anctil B, Vasilevskis EE. Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med. 2014;65:471-485.
31. Brooke BS, Stone DH, Cronenwett JL, et al. Early primary care provider follow-up and readmission after high-risk surgery. JAMA Surg. 2014;149(8):821-828.
32. Kashiwagi DT, Burton MC, Kirkland LL, et al. Do timely outpatient follow-up visits decrease hospital readmission rates? Am J Med Qual. 2012;27(1):11-15.
33. Ben-Assa E, Shacham Y, Golovner M, et al. Is telemedicine an answer to reducing 30-day readmission rates post-acute myocardial infarction? Telemed J E Health. 2014;20(9):816-821.
34. Viers BR, Lightner DJ, Rivera ME, et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol. 2015;68(4):729-735.


