(UroToday.com) The 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023, was host to a prostate cancer course. Dr. Alicia Morgans discussed the cardiac morbidity of androgen receptor (AR) agonists, compared to AR antagonists.
There are two broad categories of androgen deprivation therapy (ADT) to suppress testosterone levels:
- Surgical castration: Bilateral orchiectomy
- Medical castration, using either:
- Gonadotropin-releasing hormone (GnRH) receptor agonists
- GnRH agonists compete with GnRH produced in the hypothalamus and bind to receptors in the pituitary.
- This ultimately leads to a downregulation of the production of LH and testosterone.
- They are typically associated with an initial flare that usually lasts for approximately one week.
- GnRH receptor antagonists
- Gonadotropin-releasing hormone (GnRH) receptor agonists
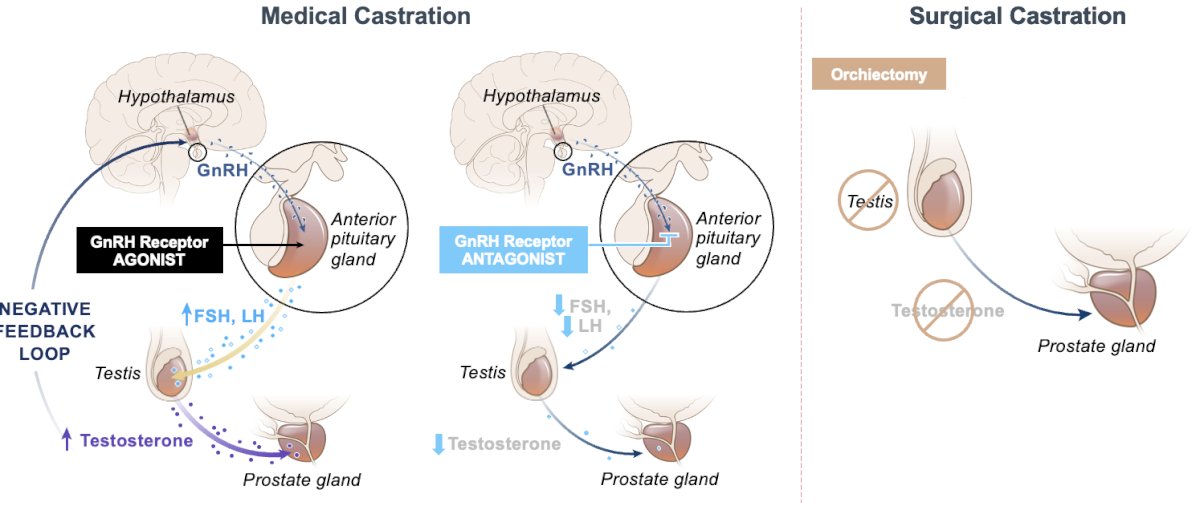
It is important to remember that hormonal therapy for prostate cancer lowers both testosterone and estrogen levels, which has many physiologic sequelae.
How do GnRH receptor agonists and antagonists differ? As summarized in the table below, GnRH antagonists are associated with a much shorter time to castration (96 hours versus 3 to 4 weeks), do not lead to a testosterone flare, and may have lower PSA failure rates. This comes at the ‘cost’ of increased frequency of local injection site reactions (40% versus 1%) and the requirement for monthly injections or daily oral pills, versus intramuscular/subcutaneous injections every three months with GnRH agonists.
While all GnRH agonists are equally effective for cancer control, GnRH antagonists may be useful in patients who require rapid reduction of testosterone levels (e.g., patients with severe symptoms or with impending emergencies – spinal-cord compression with impending paraplegia, severe bone pain, or bladder outlet obstruction).
While there remains some debate as to whether GnRH antagonists have a more favorable cardiovascular safety profile compared to GnRH agonists, what is clear is that all ADT negatively impacts cardiovascular risk factors. ADT increases total cholesterol levels, triglyceride levels, and abdominal adipose tissue and impairs glucose metabolism.1 Medical complications occur in men because of both low testosterone and low estradiol levels. Many people think of ADT as causing “male menopause” because of the associated hot flashes and changes in mood/libido. However, it is clear that the complications of ADT can significantly impact morbidity and mortality in this population.
The potential mechanisms of ADT-associated increased cardiovascular risk may differ from agonists to antagonists, with the following underlying mechanisms proposed:
- Local inflammation via activation of T cells caused by GnRH agonists triggering plaque destabilization and rupture
- Pro-inflammatory effects of Follicle Stimulating Hormone (FSH) and FSH-mediated alterations in adipocyte composition and adipokine release, inducing cardiometabolic changes
- Cardioprotection provided by testosterone interrupted by ADT (both forms)
In 2014, Albertsen et al. demonstrated that degarelix (GnRH antagonist) was associated with a lower cardiovascular risk, compared to GnRH agonists, in a cohort of patients with a history of cardiovascular disease (~30%). As demonstrated in the Kaplan Meier curve below, patients receiving degarelix had a significantly lower rate of severe cardiovascular events or death (HR: 0.44, 95% CI: 0.26 – 0.74).2

These results have been similarly corroborated by Margel et al. who demonstrated in an open-label, phase 2 trial that GnRH antagonists were associated with lower risk of cardiovascular events or major cardiovascular and cerebrovascular events.3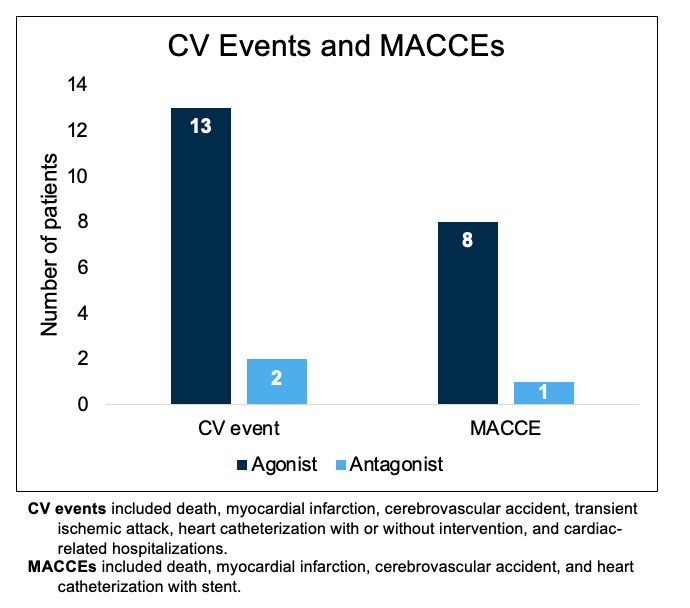
A subsequent systematic review and meta-analysis demonstrated that there was a significant reduction in both cardiovascular events and cardiovascular death with GRH antagonists, compared to GnRH agonists.4
In 2020, the phase 3 HERO trial of relugolix (oral GnRH antagonist) versus leuprolide (GnRH agonist) was published in The New England Journal of Medicine. This was a multinational, phase III, randomized, open-label, parallel-group study to evaluate the safety and efficacy of relugolix in men with advanced prostate cancer. The primary endpoint was sustained castration through 48 weeks, defined as <50 ng/dL.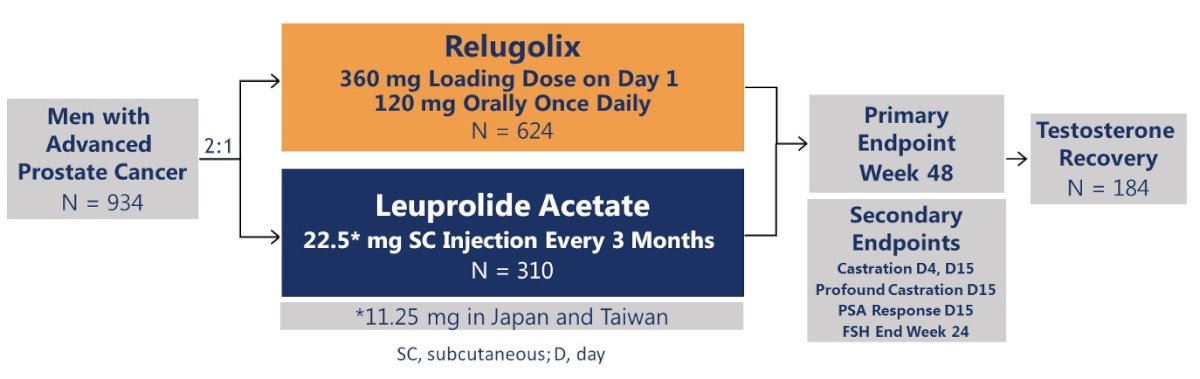
Notably, 93% of patients in this trial had pre-existing cardiovascular risk factors.
Of men who received relugolix, 97% maintained castration through 48 weeks, as compared with 89% of men receiving leuprolide. The difference of 7.9 percentage points (95% CI: 4.1 to 11.8) showed both the non-inferiority and superiority of relugolix (p<0.001 for superiority).
Significantly, patients in the relugolix arm had a 54% reduction in the risk of major adverse cardiovascular events.
This benefit was most pronounced in patients with a history of a prior major adverse cardiovascular event (3.6% versus 17.8%).5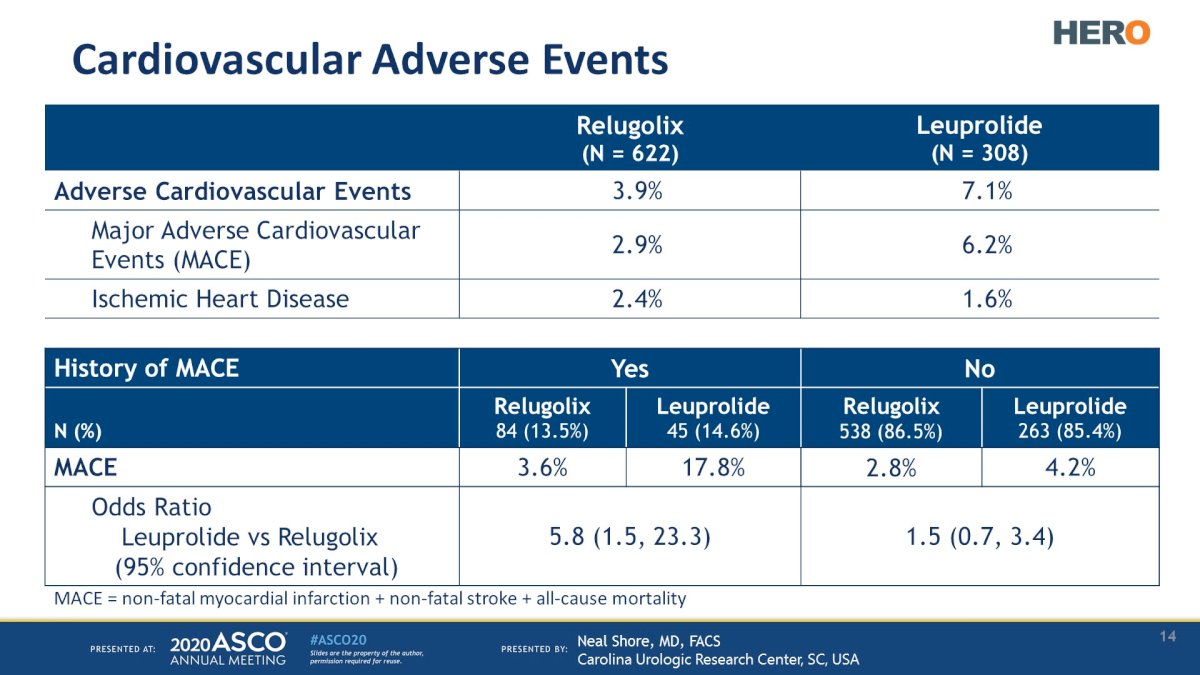
In 2021, results of the PRONOUNCE randomized trial comparing the cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer were published. No significant differences were observed between patients treated with degarelix (5.5%) versus leuprolide (4.1%; HR: 1.28, 95% CI: 0.59 – 2.79, p=0.53). However, there were several significant limitations to this trial. The recruitment was slower than originally planned, with the primary outcome event rate lower than expected. The sponsor closed enrollment with 545 of the planned 900 participants recruited.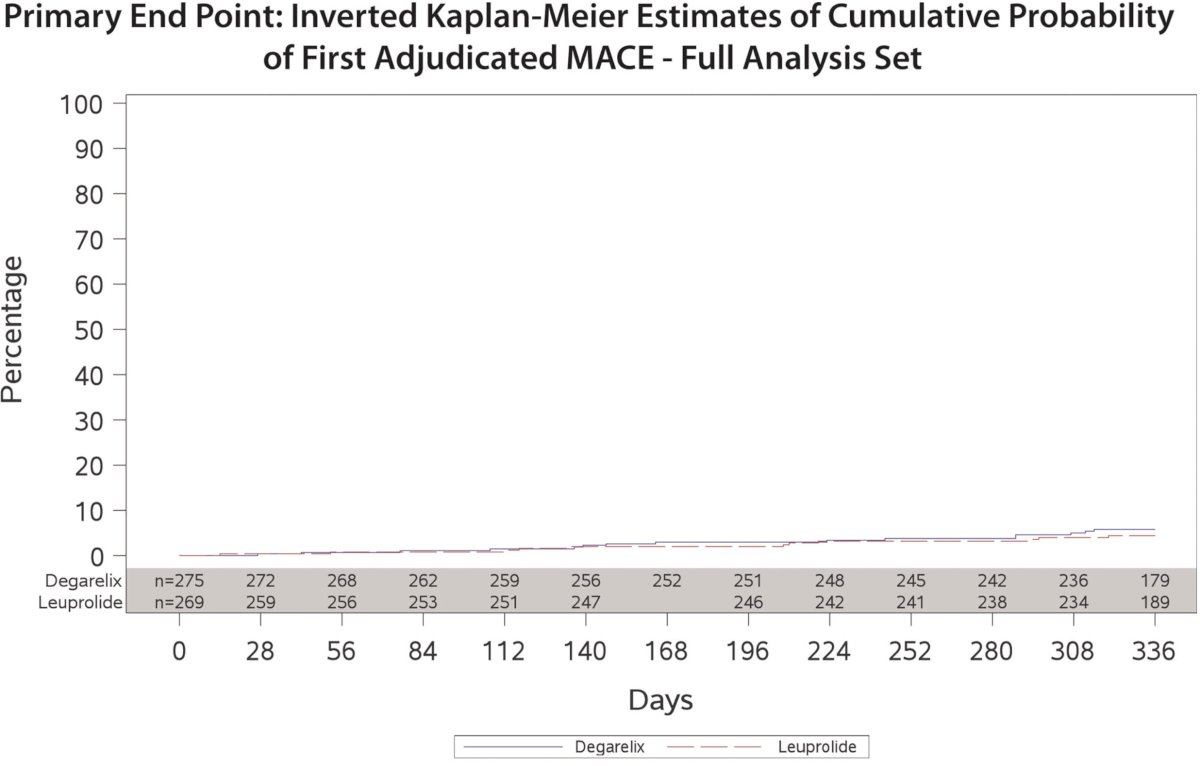
The RADICAL PC study prospectively characterized 2,492 consecutive men (mean age 68 years) with prostate cancer (newly diagnosed or with a plan to ADT for the first time) from 16 Canadian sites. Cardiovascular risk was estimated by calculating Framingham risk scores. Most (58%) were current or former smokers, 22% had known cardiovascular disease, 16% diabetes, 45% hypertension, 31% body mass index 30 kg/m2 or greater, and 24% had low levels of physical activity. Interestingly, patients with more cardiovascular risk factors were more likely to receive ADT in this study. This may have been driven by greater age and lower socioeconomic status in the group with higher risk prostate cancer who were planned to get ADT.6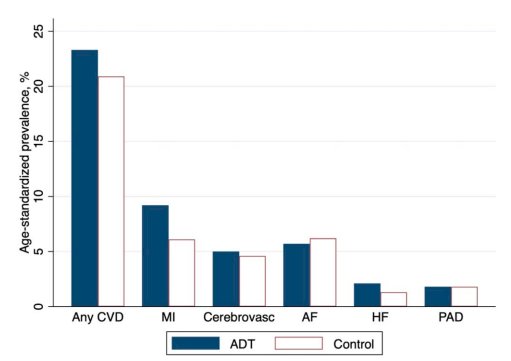
The NCCN guidelines currently recommend the ‘ABCDE’ systematic approach to addressing cardiovascular risk factors in cancer patients, as summarized below: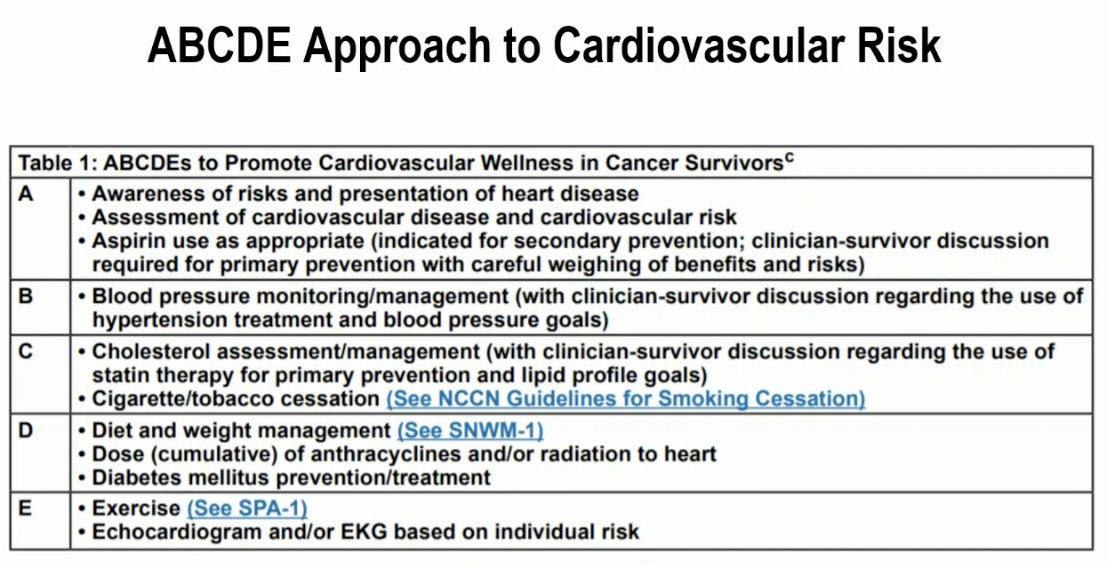
Dr. Morgans concluded her presentation with the following take home messages:
- Pharmacologic ADT can be delivered as GnRH agonist or antagonist therapy
- Differences between these include mode of administration and testosterone flare, but disease control is similar
- ADT negatively affects cardiovascular risk factors and is likely associated with risk of cardiovascular events
- GnRH antagonists may be associated with lower risk of cardiovascular events when compared with GnRH agonists
- Systematic management of cardiovascular risk factors is a recommended approach to providing optimal cardiovascular care for patients
Presented by: Alicia K. Morgans, MD, MPH, Associate Professor, Department of Medicine, Medical Director of the Survivorship Program at Dana-Farber Cancer Institute, Massachusetts General Hospital, Boston, MA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Urologic Oncology (SUO) annual meeting held in Washington, D.C. between November 28th and December 1st, 2023
References:- Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009;181(5):1998-2006.
- Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565-573.
- Margel D, Peer A, Ber Y, et al. Cardiovascular Morbidity in a Randomized Trial Comparing GnRH Agonist and GnRH Antagonist among Patients with Advanced Prostate Cancer and Preexisting Cardiovascular Disease. J Urol. 2019;202(6):1199-1208.
- Cirne F, Aghel N, Petropoulos J, et al. The cardiovascular effects of gonadotropin-releasing hormone antagonists in men with prostate cancer. Eur Heart J Cardiovasc Pharmacother. 2022;8(3):253-262.
- Shore ND, Saad F, Cookson MS, et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N Engl J Med. 2020;382(23):2187-2196.
- Leong DP, Fradet V, Shayegan B, et al. Cardiovascular Risk in Men with Prostate Cancer: Insights from the RADICAL PC Study. J Urol. 2020;203(6):1109-1116.


