(UroToday.com) The Society of Urologic Oncology (SUO) annual winter meeting included an advanced prostate cancer session and a presentation by Dr. Ale Berlin discussing metastasis-directed therapy for oligorecurrent prostate cancer. Dr. Berlin started with a clinical case example of a 71 year-old-male with a diagnosis of unfavorable intermediate risk prostate cancer who underwent a radical prostatectomy + pelvic lymph node dissection in January 2011. Pathology showed Gleason score 4+3 disease, pT2N0M0 with negative surgical margins. His post-operative PSA was 0.32 ng/mL and he underwent salvage radiotherapy + 6 months of ADT in July 2012. By 2017, his PSA had increased to 1.1 ng/mL, with a PSA doubling time of 11 months. At this point, conventional imaging was negative, however a DCFPyL PET showed oligorecurrent prostate cancer in the left common iliac lymph node:
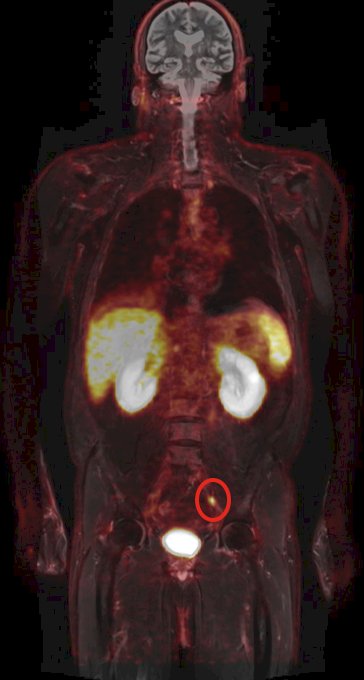
Dr. Berlin notes that there are several treatment alternatives for oligorecurrent prostate cancer, including:
- Continued surveillance
- Local therapy/metastasis-directed therapy along
- Stereotactic body radiotherapy to PET-avid foci
- Elective nodal irradiation with dose-intensification to miN+ disease
- Metastasis-directed therapy + short-term ADT
- ADT alone
- Next-generation systemic therapy (ADT + ARAT, chemotherapy, etc)
Dr. Berlin then continued his presentation discussing several notions as it portends to metastasis-directed therapy for oligorecurrent prostate cancer. The first notion is the ‘treachery of images’, specifically we do not see oligorecurrent disease, we see the image representation of oligorecurrent disease. A 1995 editorial suggested that ‘cancer comprises a biological spectrum, extending from when a disease is localized to one that is systemic when first detectable but with many intermediate states.’ Furthermore, ‘an attractive consequence of the oligometastatic state is that some patients should be amenable to a curative therapeutic strategy.’
When assessing an oligometastatic lesion, it is important to characterize and classify this lesion, which is feasible with the following questions: (i) Is there a history of polymetastatic disease? (ii) Is there a history of oligometastatic disease? (iii) Is it > 6 months from the primary date of diagnosis? (iv) Is the patient undergoing active systemic treatment? (v) Are there any oligometastatic lesions progressing? The rational for metastasis-directed therapy is that metastases can metastasize, which resistance to current systemic therapies inevitably emerges. Thus, the addition of local therapy at metastatic foci might delay lethal disease progression and if ‘all encompassing,’ eradicate all disease.
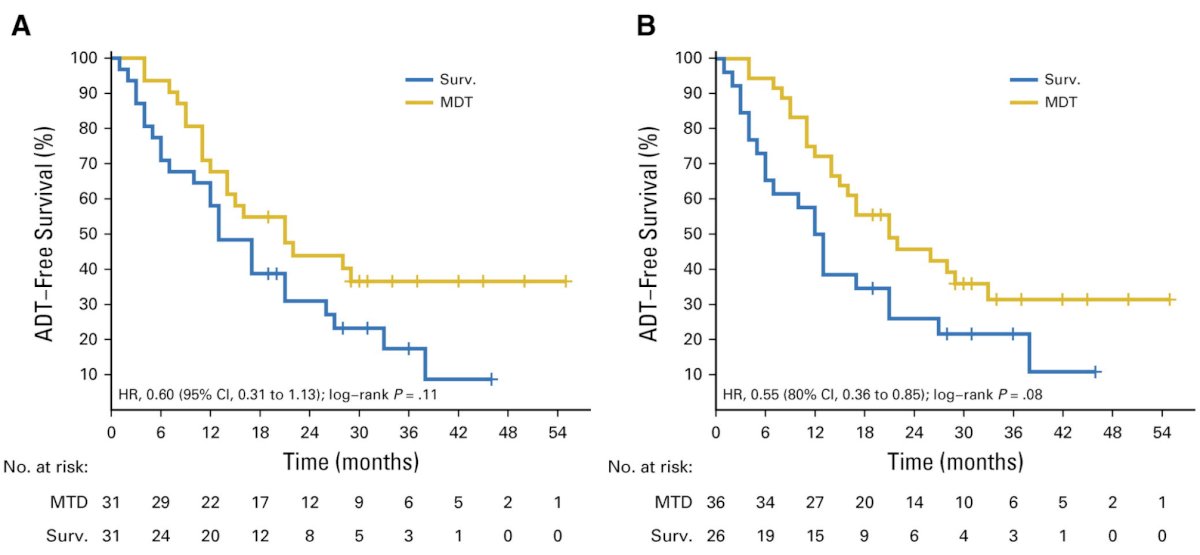
Over the last several years there have been phase 2 trials that have published results regarding metastasis-directed therapy for metastasis-directed therapy. In the STOMP trial, Ost and colleagues [1] randomly assigned 62 patients to either surveillance or metastasis-directed therapy of all detected lesions (surgery or stereotactic body radiotherapy), with a primary end point of ADT-free survival. At a median follow-up time of 3 years (IQR 2.3-3.75 years), the median ADT-free survival was 13 months (80% CI 12 to 17 months) for the surveillance group and 21 months (80% CI 14 to 29 months) for the metastasis-directed therapy group (HR 0.60, 80% CI 0.40 to 0.90; log-rank p = 0.11):
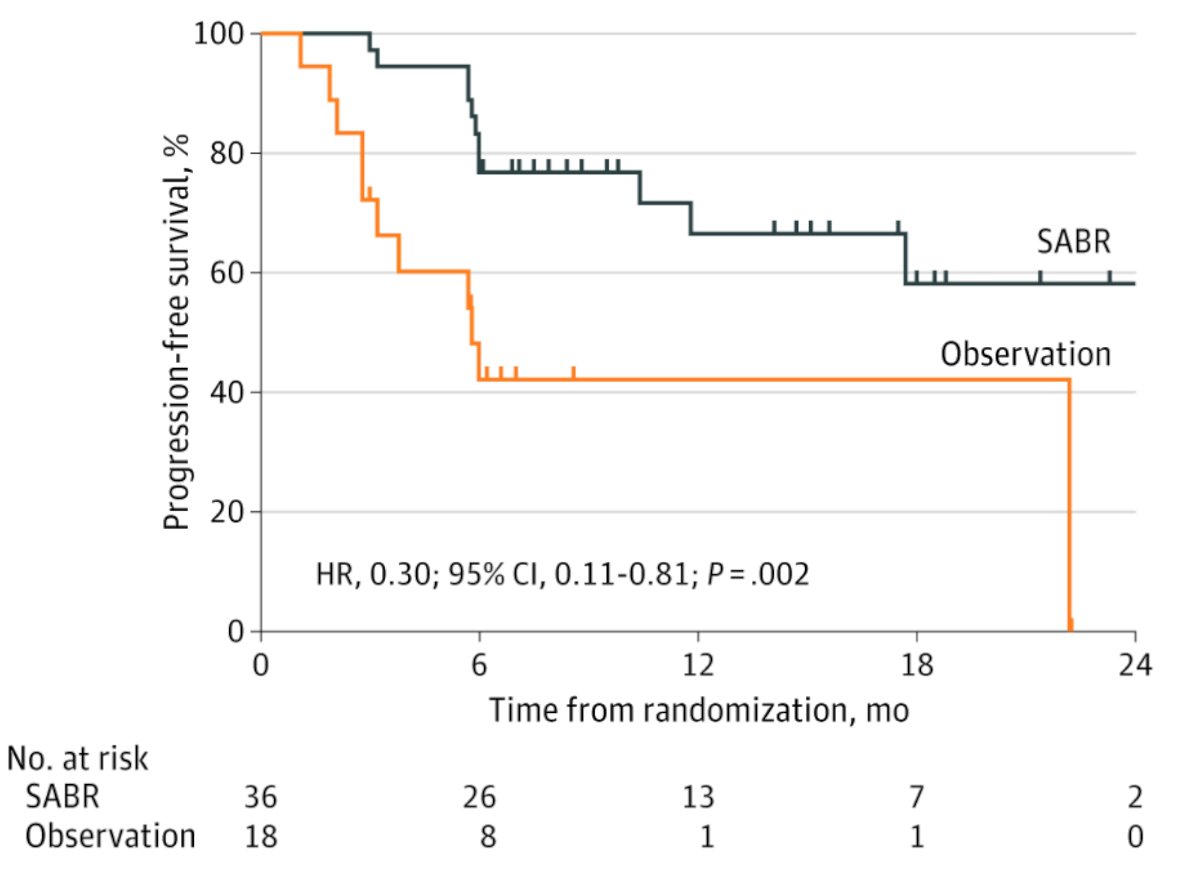
The second phase II trial published recently was the ORIOLE trial [2], randomizing 54 men in a 2:1 ratio to receive stereotactic body radiotherapy or observation. The primary endpoint for this trial was progression at 6 months, defined as a PSA increase, radiographic or symptomatic progression, ADT initiation, or death. Progression at 6 months occurred in 7 of 36 patients (19%) receiving stereotactic body radiotherapy and 11 of 18 patients (61%) undergoing observation (p = 0.005). Furthermore, treatment with stereotactic body radiotherapy improved median progression-free survival (not reached vs 5.8 months; HR 0.30, 95% CI 0.11-0.81; p = 0.002):
For those patients in the stereotactic body radiotherapy arm that had a PSMA PET-CT scan, the proportion of men with disease progression at 6 months was 5% in those who did not have any untreated lesions, compared to 38% in those who did have some untreated PSMA avid lesions (p=0.03).
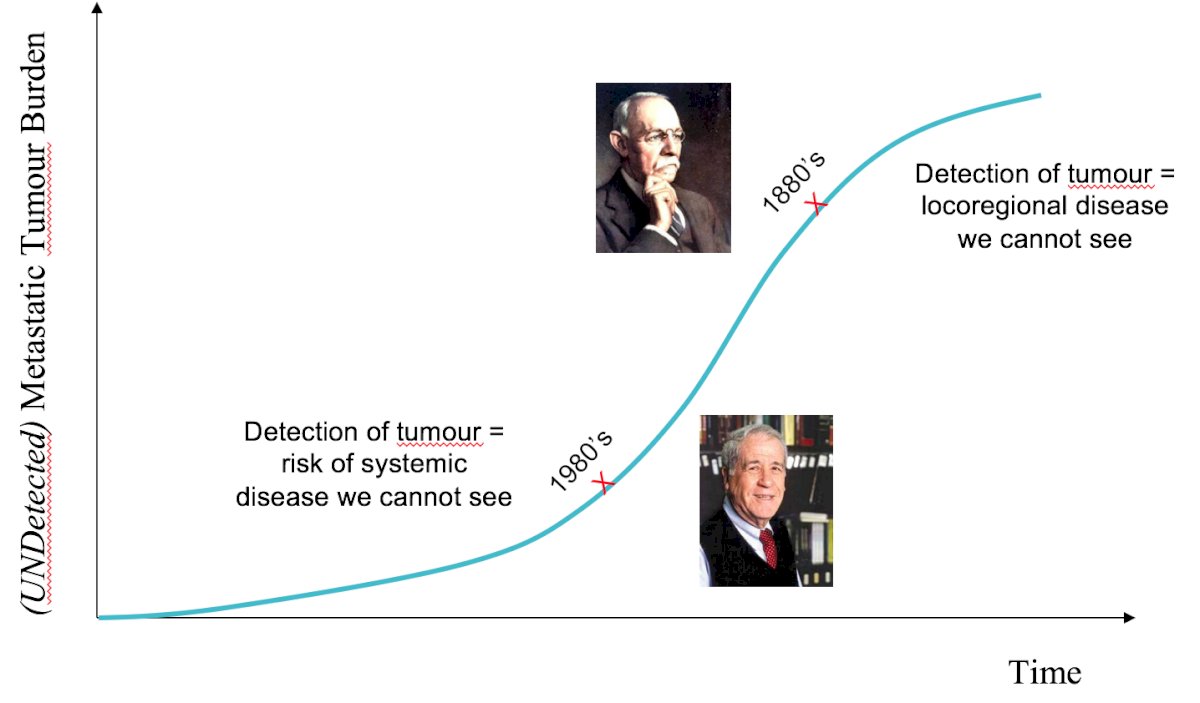
The second notion discussed by Dr. Berlin is that ‘paradigms should evolve over time,’ specifically, better detection enables intervention earlier in the natural history of the disease, as highlighted in the following figure:

With regards to targeting beyond metastasis-directed therapy, Dr. Berlin discussed OLIGOPELVIS GETUG P07 [3], which was a phase II trial of combined high-dose intensity-modulated radiotherapy and ADT (6 months) in oligorecurrent (five or fewer) pelvic node relapses in prostate cancer, detected by fluorocholine PET-CT imaging. There were 67 patients enrolled at 15 centers, of which half of the patients had received prior prostatic irradiation. After a median follow-up of 49.4 months, the median progression-free survival was 45.3 months:

The median biochemical relapse-free survival (BRFS) was 25.9 months and the median time-to-ADT was 51.9 months. There were 10% of patients that had Grade >=2 genitourinary toxicity and 2% for gastrointestinal toxicities.
Currently, there is a lot of active research in this disease space, as highlighted by the following trials:
- NRG GU011: a phase 2 randomized trial of SBRT +/- 6 months of relugolix (n=260)
- DART: a phase 2 randomized trial of SBRT +/- 6 months of darolutamide (n=128)
- RADIOSA: a phase 2 randomized trial of SBRT +/- 6 months of LHRH agonist (n=150)
- RAVENS: a phase 2 randomized trial of SBRT +/- Radium-223 (n=64)
- STORM: a phase 2 randomized trial of metastasis-directed therapy + 6 months of ADT +/- elective nodal irradiation (n=178)
The third notion is that ‘paradigms must evolve over time,’ such that advances in detection also have therapeutic implications with regards to undetected disease:
Dr. Berlin then discussed a phase 2 trial from his group assessing curative-intent metastasis-directed therapies for molecularly-defined oligorecurrent prostate cancer [4]. The hypothesis for this trial was that PSMA-targeted PET-MR/CT allows for earlier detection and localization of oligorecurrent-prostate cancer, revealing a molecularly-defined state amenable to curative-intent metastasis-directed treatment. Patients were eligible if they had a rising PSA (0.4-3.0 ng/mL) after maximal local therapy (radical prostatectomy and post-operative radiotherapy), negative conventional staging, and no prior salvage hormonal therapy. All patients underwent [18F]-DCFPyL PET-MR/CT, and those with molecularly-defined oligorecurrent-prostate cancer had stereotactic ablative body radiotherapy or surgery without hormone therapy. The primary endpoint for this trial was biochemical response defined as (i) complete (biochemical ‘no evidence of disease’; 100% PSA decline), or partial response (≥50% PSA decline from baseline) after metastasis-directed therapy. Among 72 patients enrolled, 38 (53%) had PSMA-detected oligorecurrent prostate cancer amenable to metastasis-directed therapy. Over a median follow-up of 15.9 months (IQR 9.8-19.1), among those patients receiving metastasis-directed therapy, the overall response rate was 60%, including 22% rendered biochemical no evidence of disease:

Dr. Berlin notes that it would be great to imagine a new treatment (metastasis-directed therapy) that: (i) ‘stalls’ progression for 1-2 years in patients with metastasis, (ii) has low rates of low grade toxicity (~0% Grade 5), (iii) in a small proportion of patients no other treatment is ever required, (iv) after 1-2 years, disease requires a new line of therapy (as it otherwise would), (v) no subsequent ‘standard’ therapy has been precluded, and (vi) the cost is <<< $100K/year.
Dr. Berlin concluded his presentation of metastasis-directed therapy for oligorecurrent prostate cancer with the following take home messages:
- It is feasible, well-tolerated and prolongs the ADT-free interval (ie. quality of life)
- It can render long-term, disease-free intervals in a subset (<20%) of patients
- Whether there is a need for combinatorial treatment (ie. ADT or elective nodal irradiation) to encompass undetected disease is an open question and an area of active research
- This is a disease state that was created and conditioned by imaging sensitivity, which is an evolving paradigm
- Heterogeneity is undisputable, but non-actionable
- Clinical trials remain a must, as we must disprove hypotheses with data
Presented by: Alejandro Berlin, MD, MSc, Radiation Oncologist, Princess Margaret Cancer Centre, University of Toronto, Toronto, Ontario, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Society of Urologic Oncology (SUO) Winter Annual Meeting, Orlando, FL, Wed, Dec 1 – Fri, Dec 3, 2021.
References:
- Ost P, Reynders D, Decaestecker K, et al. Surveillance of Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018 Feb 10;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020 Mar 26;6(5):650-659.
- Supiot S, Vaugier L, Pasquier D, et al. OLIGOPELVIS GETUG P07: A Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur Urol. 2021 Oct;80(4):405-414.
- Glicksman RM, Metser U, Vines D, et al. Curative-intent Metastasis-directed therapies for molecularly-defined Oligorecurrent prostate cancer: A Prospective phase II trial testing the oligometastasis hypothesis. Eur Urol. 2021;80(3):374-382.


