(UroToday.com) The Society of Urologic Oncology (SUO) annual winter meeting included a penile cancer session and a presentation by Dr. Srikala Sridhar discussing novel systemic therapeutic approaches in penile cancer. Dr. Sridhar started by highlighting that penile cancer is a rare malignancy, comprising <0.5% of all male cancers, with a significant geographic variation.
Risk factors include HPV infection, phimosis, smoking, and chronic inflammation, with the most common histology being squamous cell carcinoma. A key predictor of overall survival is nodal status, and at diagnosis, ~50% will have inguinal adenopathy. As based on SEER population-level data, outcomes for distant disease are dismal with a 5-year relative survival rate of 9%.
Systemic therapy in metastatic disease is typically TIP or cisplatin + 5FU, with response rates of 15-55% and a median overall survival of 5-12 months. However, responses are not durable and toxicity is a major concern. Furthermore, there are no standard second-line options, with a second-line median overall survival (OS) of <6 months. As follows is a summary of the systemic chemotherapy options for metastatic disease:

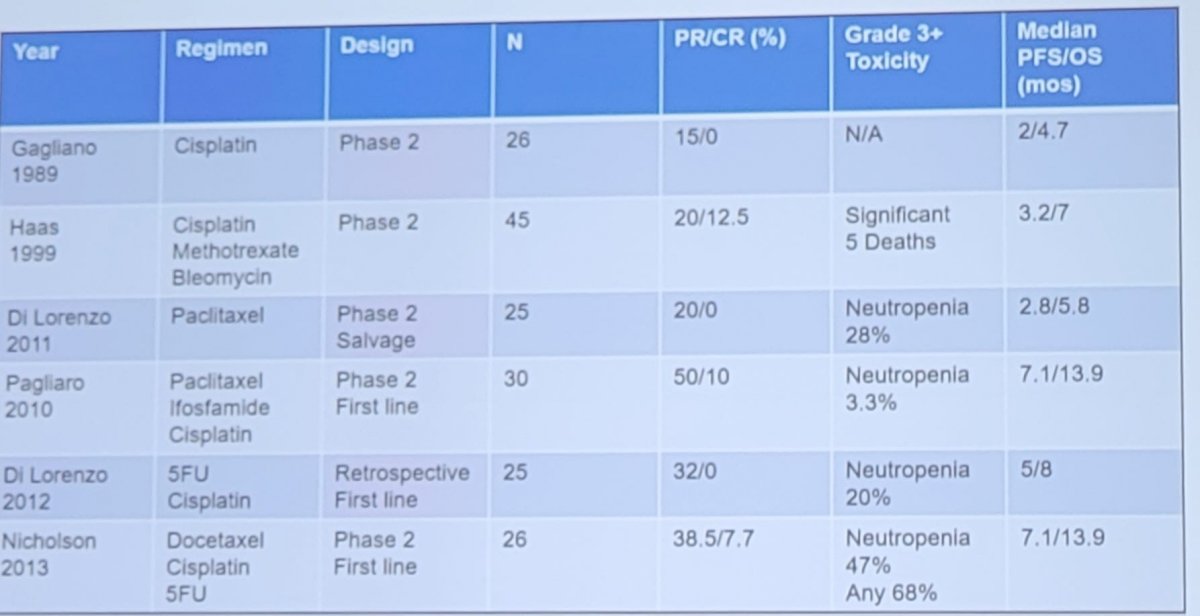
Trials of systemic chemotherapy have been small, with few complete responses, high toxicity, and low durability. As such, there is a significant unmet need for novel therapeutic approaches. As follows is a table summary of these trials:
Recently published was the VinCaP phase 2 trial,1 which assessed the first-line activity of vinflunine in patients with penile cancer. There were 25 patients with inoperable squamous carcinoma of the penis that was recruited to this single-arm trial and treated with 4 cycles of vinflunine 320 mg/m2, given every 21 days. Among 22 evaluable patients, 10 objective responses or disease stabilizations were confirmed. The primary endpoint was the clinical benefit rate, which was 45.5%, and the partial response rate was 27.3%. The median progression-free survival was 2.9 months (95% CI 1.4-6.4 months):
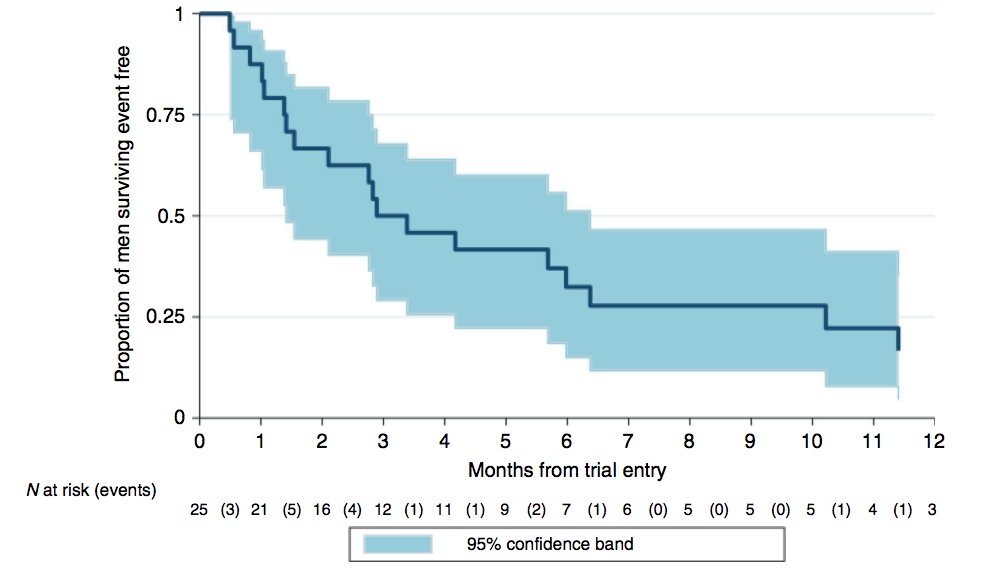
The median overall survival was 8.4 months (95% CI 3.2 -14.1 months):

Seven patients received >4 cycles of vinflunine and dose reduction or treatment delay was required for 20% of cycles. Overall, 68% of patients experienced at least one grade 3 adverse event. According to Dr. Sridhar, it is encouraging to see that trials are completing accrual, and vinflunine may be a potential treatment option for patients who are not candidates for TIP chemotherapy.
Dr. Sridhar notes that, unlike other HPV-related cancers, only about 50% of penile squamous cell carcinomas are HPV-related. Thus, instigating molecular events may be different, as highlighted in the following table:

HPV is a DNA virus, with transient, asymptomatic infections. However, HPV can also persist and cause warts or precancerous lesions, integrating its genome into the nuclear DNA. HPV genotypes 16 and 18 are the most common in penile cancer, and HPV oncoproteins E6 and E7 promote transformation and tumorigenesis. Therapeutic strategies are being explored which target E6/E7, including vaccines, T-cell therapy, nucleic acid-based therapy, and programmable nucleases.
Immune checkpoint inhibitors are now approved in many cancers, including HPV-related cancers. PD-L1 is detected in ~40-60% of penile cancers, is more common in HPV negative tumors, and diffuse PD-L1 expression predicts poor survival in multivariable analyses. Immune checkpoint inhibitors may be better tolerated than standard chemotherapy and thus PD1/PD-L1 may be a rational therapeutic target in penile cancer. As of 2019, as follows is a selection of current trials of immunotherapy in penile cancer 2:

Dr. Sridhar also notes that key ongoing trials include PULSE (maintenance avelumab after response to chemotherapy) and Hercules (pembrolizumab with chemotherapy). Ultimately, trials remain challenging, requiring large-scale collaborations, and need for industry support.
Immunotherapy is not effective in all patients and response depends on several immunomodulatory factors including:
- Tumor mutational burden: comparable to cervical cancer, lower than head and neck cancer, and much lower than bladder cancer and melanoma
- Immune cell infiltration: immune desert, immune inflamed, and immune excluded
- Tregs and M2 can block the immune response
Dr. Sridhar notes that there is growing interest in “adoptive cell therapy” by removing tumor-infiltrating lymphocytes (TILs), activating, expanding, and then re-infusing. A recent pilot study in penile cancer has demonstrated the feasibility and potential therapeutic value of using adoptive cell therapy. TILs were expanded from 91.6% (11 of 12) samples of metastatic lymph nodes, and the total number of TILs expanded from tumor samples were not impacted by prior neoadjuvant chemotherapy or HPV positivity status.
There may also be a role for targeted therapies in penile cancer. Comprehensive genomic profiling has previously demonstrated that EGFR amplification is present in 20% of tumors, PIK3CA alterations in 25%, CDKN2A alterations in 40%, and CCND1 amplification in 20%. Similar alterations have also been demonstrated in ctDNA, thus it may be feasible to do non-invasive continuous monitoring.
Dr. Sridhar concluded her presentation of novel systemic therapeutic approaches in penile cancer with the following take-home messages:
- Penile cancer is a rare malignancy with significant unmet needs
- Clinical trial accrual and translational research has been challenging
- Recent advances in chemotherapy, immunotherapy and targeted therapy hold promise for better outcomes in this disease
- Large scale, multidisciplinary collaborations, as evidenced by the InPACT trial, will be key
- Dedicated strategies to boost accrual to this and other ongoing trials will be of paramount importance
- There is also a need to bring experts in the field together, through organizations like the International Rare Cancers Initiative and the Global Society of Rare Genitourinary Tumors, to design and develop the next round of trials
Presented by: Srikala Sridhar, MD, MSc, FRCPC, Medical Oncologist, Princess Margaret Cancer Centre, University of Toronto, Toronto, Ontario, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Society of Urologic Oncology (SUO) Winter Annual Meeting, Orlando, FL, Wed, Dec 1 – Fri, Dec 3, 2021.
References:
- Nicholson S, Tovey H, Elliot T, et al. VinCaP: A phase II trial of vinflunine in locally advanced and metastatic squamous carcinoma of the penis. Br J Cancer. 2021 Oct 20 [Epub ahead of print].
- de Vries HM, Ottenhof SR, Horenblas S, et al. Defining the tumor microenvironment of penile cancer by means of the cancer immunogram. Eur Urol Focus. 2019;5:718-721.


