(UroToday.com) At this year's Society of Urologic Oncology (SUO) annual meeting Vadim S. Koshkin presented on systemic therapy for metastatic urothelial carcinoma. The current state of first-line metastatic urothelial carcinoma is shown in Figure 1.
Figure 1 – Current state of first-line treatment of metastatic urothelial carcinoma:

In recent years, there have been several front-line clinical trials in metastatic urothelial carcinoma (Figure 2).
Another important study that needs to be mentioned is the landmark JAVELIN bladder 100 study1 randomizing metastatic bladder cancer patients after first-line chemotherapy to either best standard of care or best standard of care + Avelumab switch maintenance therapy once every 2 weeks. This study demonstrated a clear overall survival advantage to the avelumab arm with a median overall survival advantage of more than 7 months (21.4 vs. 14.3 months, HR 0.69 [95% CI 0.56-0.86]) (Figure 3).
Figure 2 – Front-line clinical trials in metastatic urothelial carcinoma: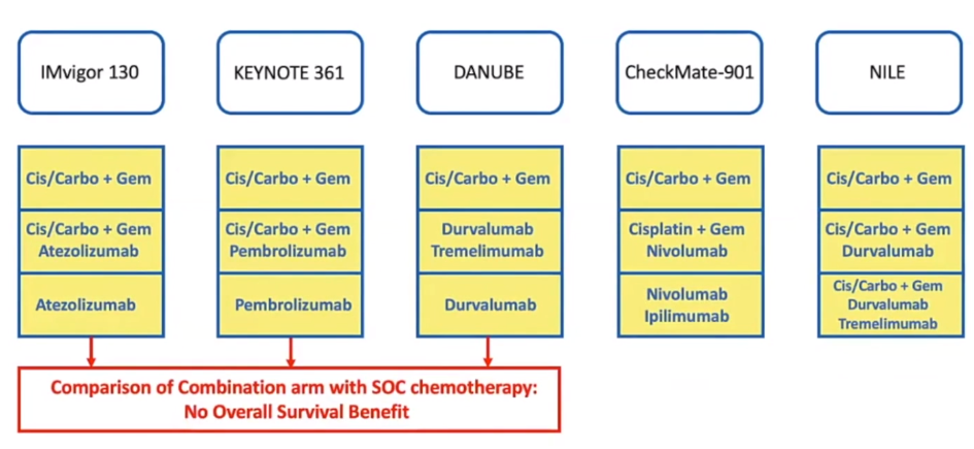
Figure 3 – The JAVELIN Bladder 100 trial: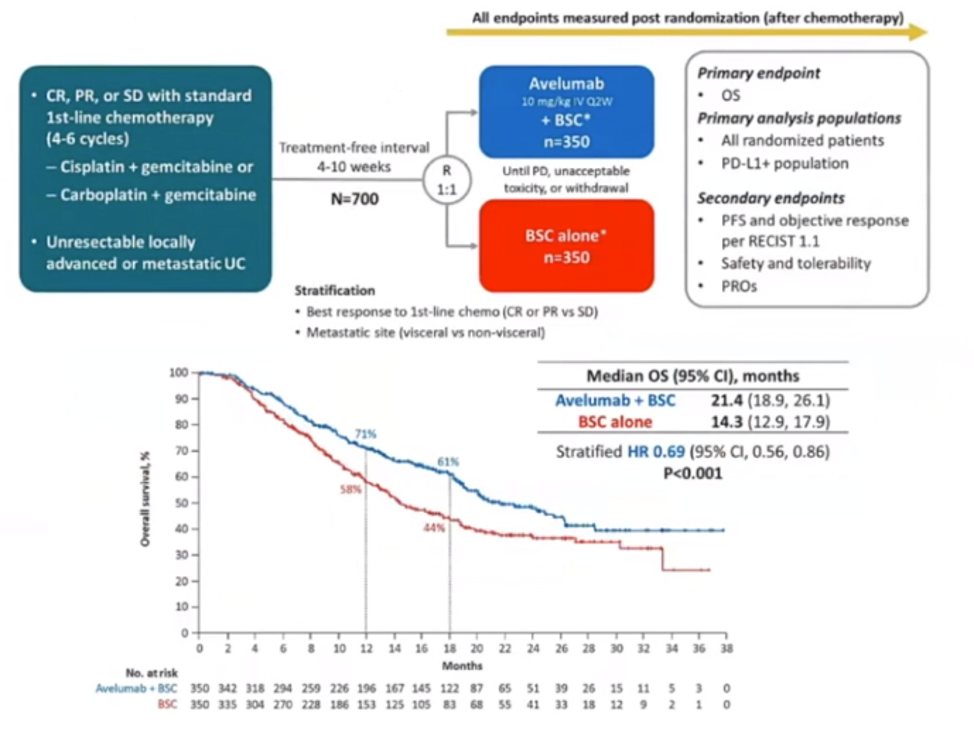
Due to the significant improvement recently seen in the survival of metastatic bladder cancer patients, it is now imperative that we think of the next logical step of 3rd line therapy (Figure 4). The available options following immunotherapy are shown in table 1, describing some of the exciting new drugs that have been recently analyzed and published in high-impact journal publications such as Enfortumab vedotin2 and Erdafitinib3, resulting in a new treatment paradigm, as shown in Figure 5.
Figure 4 – Treatment paradigm for metastatic bladder cancer patients:
Table 1- Treatment options following immunotherapy in metastatic bladder cancer patients: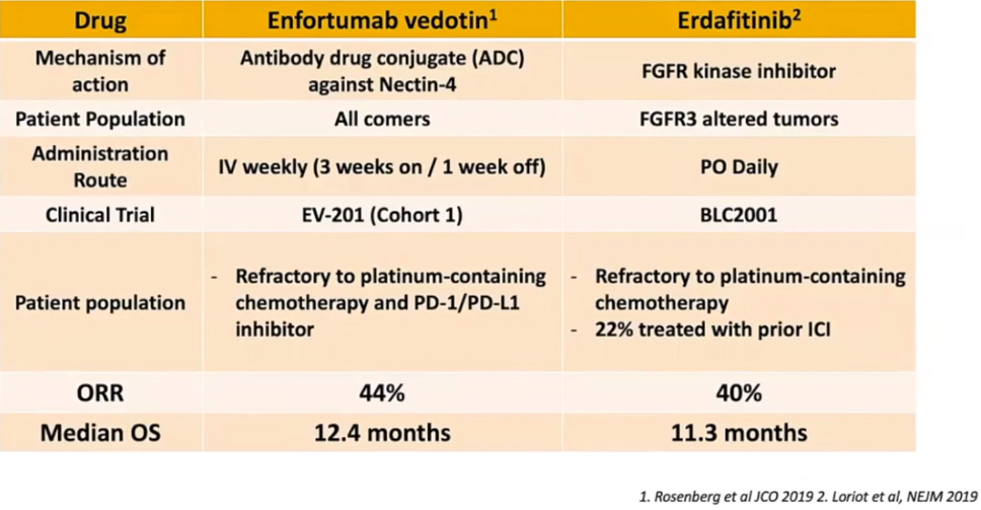
Figure 5 – New treatment paradigms for metastatic bladder cancer patients:
Next, Dr. Koshkin moved on to describe the available front-line treatment options for another important group of patients, the cisplatin-ineligible metastatic bladder cancer patients. For these patients, there are some promising results for the combination of pembrolizumab and Enfortumab vedotin with an objective response rate of 73% with 12 months overall survival rate of 81%4 (Figure 6).
Figure 6 – Selected front line trials for cisplatin-ineligible patients:
In summary, platinum-based chemotherapy remains the established front-line therapy in metastatic urothelial cancer, now followed by anti-PD-1/PD-L1. Enfortumab vedotin is an active drug in the post immunotherapy setting and moving into earlier treatment settings. Erdafitinib is available for molecularly selected platinum-refractory patients. Lastly, metastatic urothelial carcinoma treatment can inform treatment and trial design in the perioperative setting.
Presented by: Vadim S. Koshkin, MD, University of California, San Francisco
Written by: Hanan Goldberg, MD, MSc, Assistant Professor, Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, @GoldbergHanan during at the 21st Annual Meeting of the Society of Urologic Oncology (SUO), December 3-5, Virtual Conference
References:
1. Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine 2020; 383(13): 1218-30.
2. Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. Journal of Clinical Oncology 2019; 37(29): 2592-600.
3. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine 2019; 381(4): 338-48.
4. Rosenberg JE, Flaig TW, Friedlander TW, et al. Study EV-103: Preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. Journal of Clinical Oncology 2020; 38(6_suppl): 441-.


