
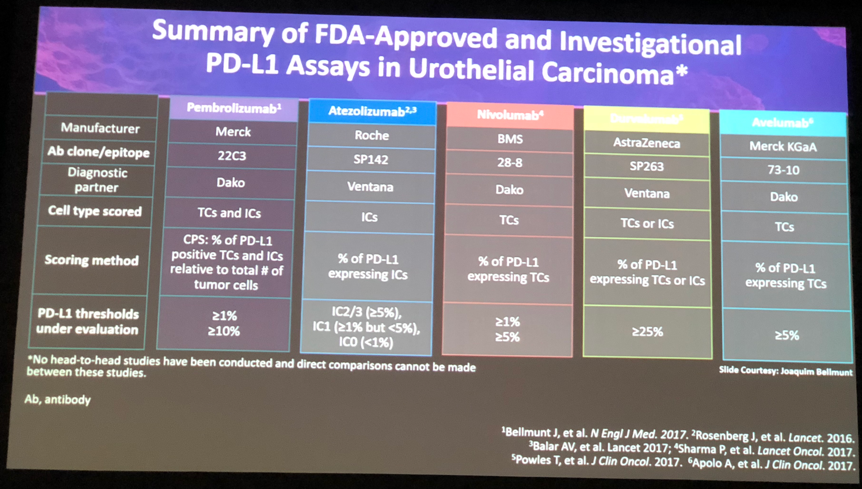
The PD-L1 expression (prognostic factor) is seen in approximately 20-30% of specimens and it’s associated with the increased pathologic stage, increased all-cause mortality and more aggressive disease. Durvalumab correlates with PD-L1 high expression with greater efficacy (High PD-L1, objective response rate of 31% VS 5.1% of low/negative PD-L1, but efficacy is still observed). Atezolizumab and pembrolizumab had no impact of PD-L1 status on overall survival.
In the IMvigor 210 trial (atezolizumab) was found that basal clusters had the highest prevalence of IC 2/3 PD-L1 (60 VS 23%) and TC 2/3(39 VS 8%), but the highest response was in luminal cluster II subtype (ORR=34%, p=0.0017).
The tumor mutational burden (neoantigen burden) is associated with a greater likelihood of durable responses to immune checkpoint blockade. Neoantigen burden predicts response more robustly than PD-L1 and presence of TILs. IMvigor 210: cohort II found a higher mutational load in responding vs non-responding patients (12.4 VS 6.4 per megabase, p<0.0001).
Multiparameter immune gene expression profiling in the Checkmate 275 study (nivolumab) found IFN-γ signature correlated with better response to nivolumab (high IFN-γ signature: CR/Prin 20/59 patients; medium or low IFN-γ signature: CR/PR in 18/118 patients; p=0.0003).
Kamat concluded that PD-L1 positivity inconsistently enriches for clinical benefit, TCGA and other subtypes have varied associations, tumor mutational burden correlates with response and immune gene expression profiles studies still ongoing for findings.
Presented by: Ashish M. Kamat, MD, Professor of Urologic Oncology, MD Anderson Cancer Center, Houston, TX
Written by: Eduardo Gonzalez-Cuenca, MD, Urology Resident, Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, Mexico City and Ashish M. Kamat, MD, Professor of Urologic Oncology, MD Anderson Cancer Center, Houston, TX at the 2018 Congreso de la Asociación Mexicana de Urología Oncológica – July 25-28, 2018, Acapulco, GRO México


