(UroToday.com) The Société Internationale D’Urologie (SIU) 2021 annual meeting included a plenary session with a State-of-the-Art lecture by Dr. Sia Daneshmand discussing new markers and the changing face of testicular cancer. Dr. Daneshmand started by highlighting that there are several challenges in testicular cancer, with several potential solutions:
- Stage I
- Current challenges: 50-80% of patients are cured with orchiectomy, but the challenge is identifying those that are going to relapse early
- Potential solutions: surveillance for most of stage I, plus miRNA 371 assisting as a new biomarker
- Stage IIA/B
- Current challenges: 25-40% are pathology stage I disease and are over-treated with chemotherapy/radiation
- Potential solutions: RPLND for early metastatic seminoma and NSGCT to avoid long term toxicity of systemic therapy
- Post-chemotherapy seminoma
- Current challenges: 80-90% have fibrosis, PET scan has a high false-positive rate, and identification of those with viable residual disease is difficult
- Potential solutions: miRNA 371
- Post-chemotherapy NSGCT
- Current challenges: 45% have fibrosis in residual masses >1 cm, and it is difficult to identify those with teratoma or viable disease
- Potential solutions: miRNA 371 and a better marker for teratoma (possibly miRNA 375)
- All stages
- Current challenges: Misdiagnosis leading to under treatment/over treatment
- Potential solutions: referral/consultation with expert centers
Dr. Daneshmand notes that according to the NCCN guidelines for clinical stage I NSGCT, for those without risk factors active surveillance is preferred and is an option for those with risk factors. This is recapitulated by the AUA guidelines in that: (i) clinicians should recommend surveillance for patients with stage IA NSGCT. RPLND or BEP x1 chemotherapy is effective and appropriate alternative treatment options (Moderate Recommendation; Evidence Level: Grade B), and (ii) for patients with a stage IB NSGCT, clinicians should recommend surveillance, RPLND, or one or two cycles of BEP chemotherapy (Strong Recommendation; Evidence Level: Grade B).
For clinical stage IIA seminoma, the NCCN guidelines offer radiotherapy to include para-aortic and ipsilateral iliac lymph nodes to a dose of 30 Gy or primary chemotherapy including BEP for 3 cycles or EP for 4 cycles. However, there is long-term morbidity from chemotherapy and radiotherapy. Young patients have excellent cure rates, but also years of potential cumulative toxicities. These including: (i) cardiac events (HR 2.2-2.8), (ii) secondary cancers (hematologic, solid tumors), (iii) metabolic syndrome (~2x incidence), (iv) bleomycin-induced pulmonary injury, (v) infertility, (vi) neurotoxicity, and (vii) tinnitus. So, is there a treatment that can reduce long-term morbidity?
The SEMS trial evaluated surgery in metastatic seminoma (NCT02537548) and was first presented at GU ASCO 2021. This trial included 12 sites in the United States and Canada that prospectively enrolled patients (16 years of age or older) with testicular seminoma and isolated retroperitoneal lymphadenopathy between 1-3 cm in size. Patients were excluded if they received prior therapy (except orchiectomy) for testicular cancer or if the patient’s relapse was greater than three years from diagnosis. Open, modified-template RPLND was performed by qualified surgeons (>= 8 open RPLND in 1 year or >24 open RPLND in 3 years) with a primary endpoint of 2-year recurrence-free survival. Secondary endpoints included 5-year recurrence free survival, complication rates (short and long-term), pathologic up/downstaging, recurrence patterns, adjuvant therapies, and treatment-free survival.
A total of 55 patients were enrolled in SEMS and underwent RPLND. Fourteen patients had initial stage I disease who developed isolated retroperitoneal relapse while 41 patients had clinical stage IIA-B at presentation. The median age was 34 years of age (range: 21-64) with 80% of patients being white. With a median follow-up of 24 months (range: 8-52 months), there were a total of 10 recurrences. The overall recurrence rate was 18% with a median time to recurrence of 8 months; the two-year recurrence free survival rate was 84%. Of the recurrences, eight underwent chemotherapy (6 BEP x 3, 1 EP x 4, 1 carbo/etoposide) and two underwent additional surgery. As follows is the Kaplan-Meier curve for recurrence-free survival and treatment-free survival:
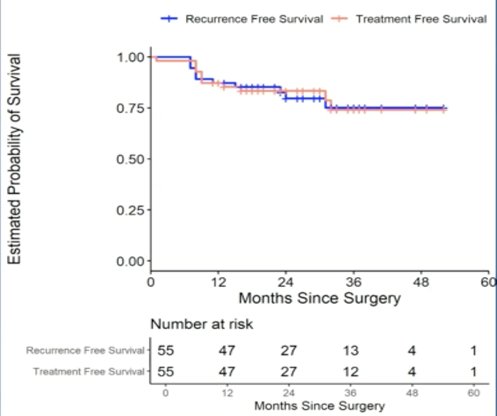
There were 7 (13%) patients who experienced short-term complications within one year of RPLND. Of these, 5 (9%) were classified as Clavien Dindo I-II and 2 (3.6%) were classified as Clavien Dindo III (chylous ascites, pulmonary embolism). No patients have reported long-term complications. Dr. Daneshmand’s conclusions from the SEMS trial were as follows: (i) the SEMS trial established RPLND as a therapeutic option as a first-line treatment in early metastatic seminoma with isolated retroperitoneal lymphadenopathy, (ii) with 2-year median follow-up, systemic treatment free survival was 84% and overall survival was 100%, and (iii) the surgery offers cancer control rates similar to those seen in non-seminomatous germ cell tumors and is an attractive option given the favorable long-term morbidity of RPLND.
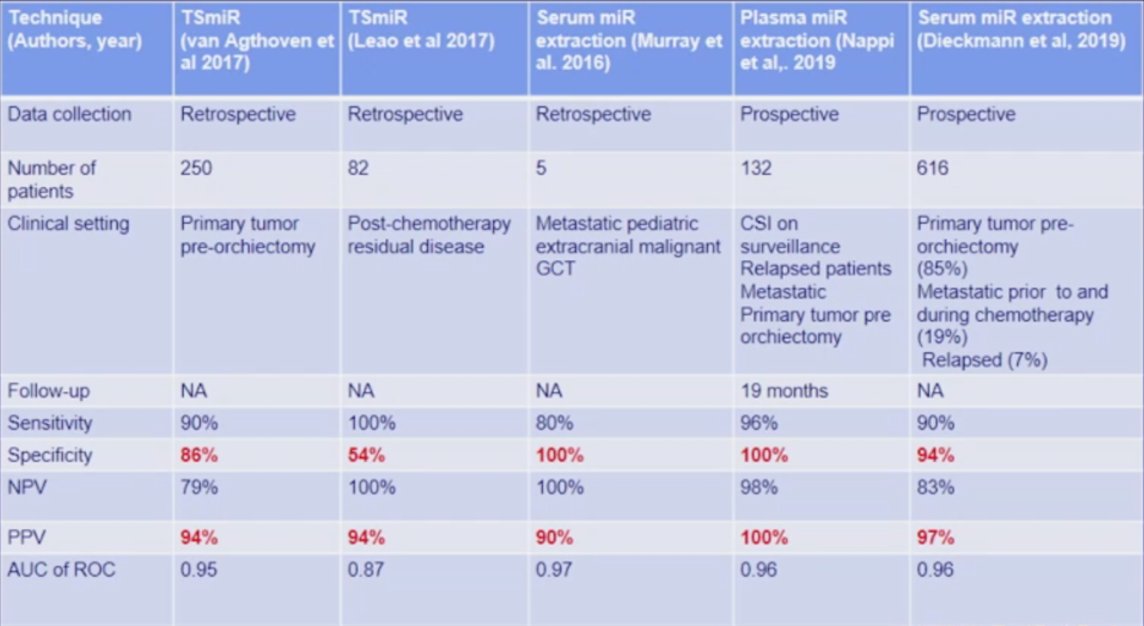
Dr. Daneshmand then discussed the role of miRNA 371, which in his opinion will change everything in the management of testicular cancer in the next 5-10 years. Micro-RNAs are small (about 22 nucleotides) non-coding RNA regulating oncogenes and oncosuppressors of transcription. Additionally, they are detected in very small volumes (50-400 ul) of serum/plasma, and expression is quantified by real-time PCR. The extraction kit, as well as the reagents for the real-time PCR, are commercially available with an estimated cost of $60 USD. With respect to germ cell tumors, miR-371 is released in the blood and is stable for months, with detection as early as GCNIS pre-surgery and in those with metastatic disease; miR-371 is also proportional to tumor burden. Based on several studies, miR-371a-3p sensitivity and positive predictive value are excellent, as highlighted in the following table:
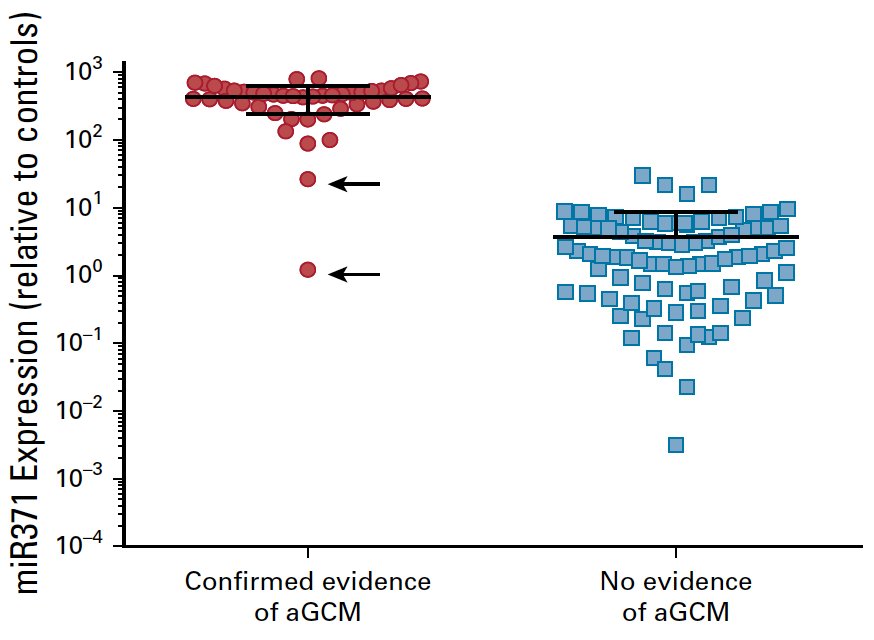
Nappi et al.1 published one of the landmark papers to date in the evaluation of miR-371, assessing specificity and positive predictive value of miR371 expression by mapping it to actual clinical events in patients with a history of germ cell tumor. Among 111 men with a history of germ cell tumor, 46 (35%) of 132 samples had clinically confirmed active germ cell malignancy over the course of management; 44 (96%) of these 46 patients had plasma miR371 expression (true positives) with no false positives. Two (4%) of 46 patients had no miRNA expression despite pathologic confirmation of active germ cell malignancy (false negatives). Plasma miR371 expression in confirmed active germ cell malignancy had a specificity, sensitivity, positive predictive value, and negative predictive value of 100%, 96%, 100%, and 98%, respectively:
There are currently two clinical trials assessing the role of miR-371 in early-stage germ cell tumors, including the SOWG S1823 and AGCT 1531 trials, as summarized in the following table:

Dr. Daneshmand and Dr. Craig Nichols are also planning a stage II trial of germ cell tumor patients with the incorporation of mi-R 371 as highlighted in the following trial schema:

Taken together, future studies are moving towards personalized medicine and miRNA-directed management of clinical stage II and post-chemotherapy patients. Dr. Daneshmand concluded his presentation with the following take-home messages:
- Generalized management:
- Stage 1 GCT surveillance
- Stage IIA/B (<= 3cm) RPLND
- Post-chemotherapy RPLND for residual masses: > 1 cm NSGCT and >3 cm PET positive plus resectable
- Midline extraperitoneal RPLND reduces surgical morbidity
- Micro-RNA 371 is a useful biomarker that has been clinically validated and will change future algorithms
- Clinical trials are planned to validate utility in equivocal clinical settings
Presented by: Sia Daneshmand, MD, Professor of Urology (Clinical Scholar), Director of Urologic Oncology, Director of Clinical Research, University of Southern California, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Société Internationale D’Urologie (SIU) Hybrid Annual Meeting, Wed, Nov 10 – Sun, Nov 14, 2021.
References:


