(UroToday.com) In the World Urological Oncology Federation (WUOF) Symposium held in conjunction with this year’s virtual Société Internationale d'Urologie (SIU) annual congress, Dr. Robert Kerbel discussed a preclinical approach to predict treatment outcomes.
He began by highlighting what he deemed a “major problem” in oncology in which more than 60% of all randomized phase III trials in oncology fail to meet their primary survival endpoint, in spite of promising results from preclinical and phase II studies. He highlighted a variety of drivers of this failure including inadequate basic science, flawed study design, suboptimal dose selection, flawed data collection and analysis, problems with study operations, and others.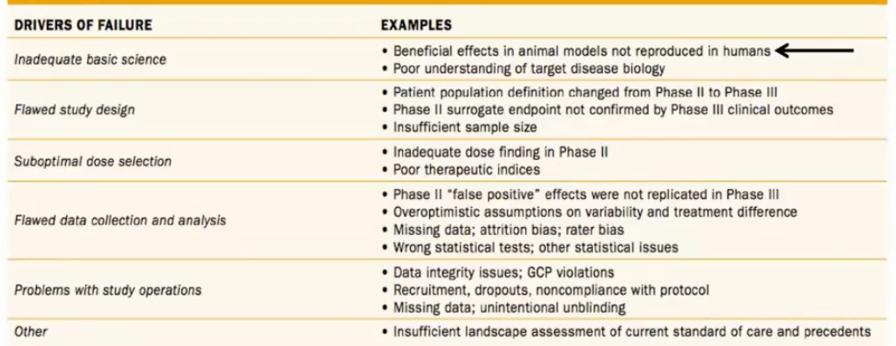
Dr. Kerbel’s focus is on the first of these issues, particularly as it relates to the beneficial effects of therapy observed in animal models failing to translate to be reproduced in human studies. To provide data that are most likely to translate into benefit in humans, Dr. Kerbel described an approach to animal research which seeks to “mimic the clinic” in mice models. In particular, as highlighted in the figure below, many animal studies rely on an approach in which a tumor is induced by various means in the mouse, followed by treatment and assessment of response, without local therapy to the tumor. This is an uncommon approach to the treatment of cancer in humans and thus, it may be expected that benefits observed in this model may not translate.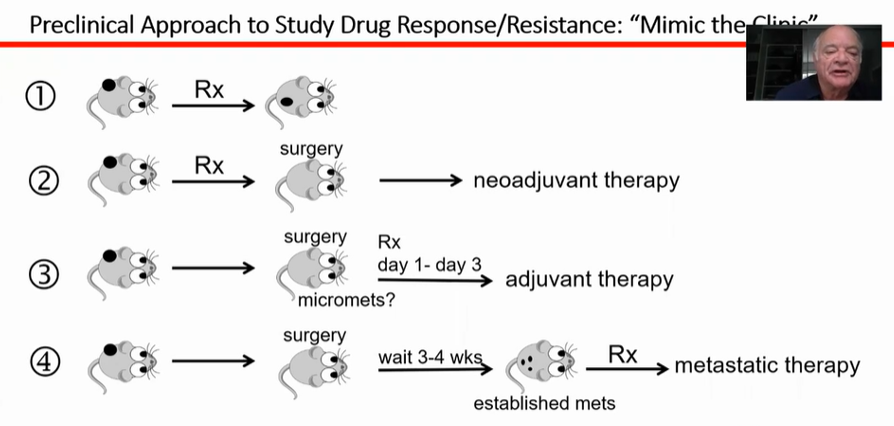
Alternatively, as highlighted in pattern #2, a neoadjuvant approach is more common in human cancer care as is the treatment of metachronous metastatic disease (pattern #4). His lab has focused on assessment of treatment approaches tested using patterns #2, 3, and 4 with a focus on anti-angiogenic VEGF pathway inhibiting results. Use of these agents has particular benefit in renal cell carcinoma, among many other sites.
Interestingly, despite proven trial successes and clinical applications of these agents in renal cell carcinoma, there have been prominent failures including attempts at combination therapy using anti-angiogenic tyrosine kinase inhibitors with chemotherapy in advanced metastatic disease and in the adjuvant setting following initial surgical extirpation. In the first category, he highlighted four combination approaches of sunitinib and chemotherapy in advanced breast cancer, each of which failed. Dr. Kerbel’s group then examined this treatment approach and distinguished the benefit of sunitinib in terms of local tumor response (as had been previously done) as compared to in a metastatic setting following initial local therapy (as is more clinically appropriate). As expected based on previous work, sunitinib demonstrated benefit in the first setting but, notably, did not have benefit in the second, more clinically relevant, study design.
Trying to understand these discrepant results, Dr. Kerbel described a process known as vessel co-option in cancer, in which tumor cells co-opt existing vasculature (such as in the lung parenchyma) rather than inducing an angiogenic process. Thus, the target of the anti-angiogenic agents is not present and we would therefore not expect benefit from use of these agents. Such a process is relatively common at sites including lung, liver, brain, and lymph nodes in which metastasis is frequent. This has been seen in breast, colorectal, and renal cell cancers which have metastasized to the lung.
In another model, Dr. Kerbel’s group injected breast cancers into mice and then performed subsequent tumor resection. Short-term use of adjuvant sunitinib in higher doses (compared to longer term, low dose approaches) was associated with no clinical benefit, and actually worse survival compared to vehicle control. In contrast, when the primary tumor was in situ, each approach demonstrated benefit. Interestingly, human adjuvant trials using anti-angiogenic agents have been unsuccessful across a variety of tumor types including colorectal cancer, breast cancer, non-small cell lung cancer, rectal cancer, melanoma, hepatocellular carcinoma, and renal cell carcinoma.
More recently, in place of anti-angiogenic therapeutics, Dr. Kerbel has been using similar models to assess immune-oncology drugs. This is in part based on the rationale that VEGF acts in an immunosuppressive manner locally. In a mouse kidney cancer model, Dr. Kerbel and colleagues examined combinations of anti-PD-L1 and sunitinib in metastatic renal cell carcinoma following initial tumor resection. A combined approach offered the greatest benefit in this setting.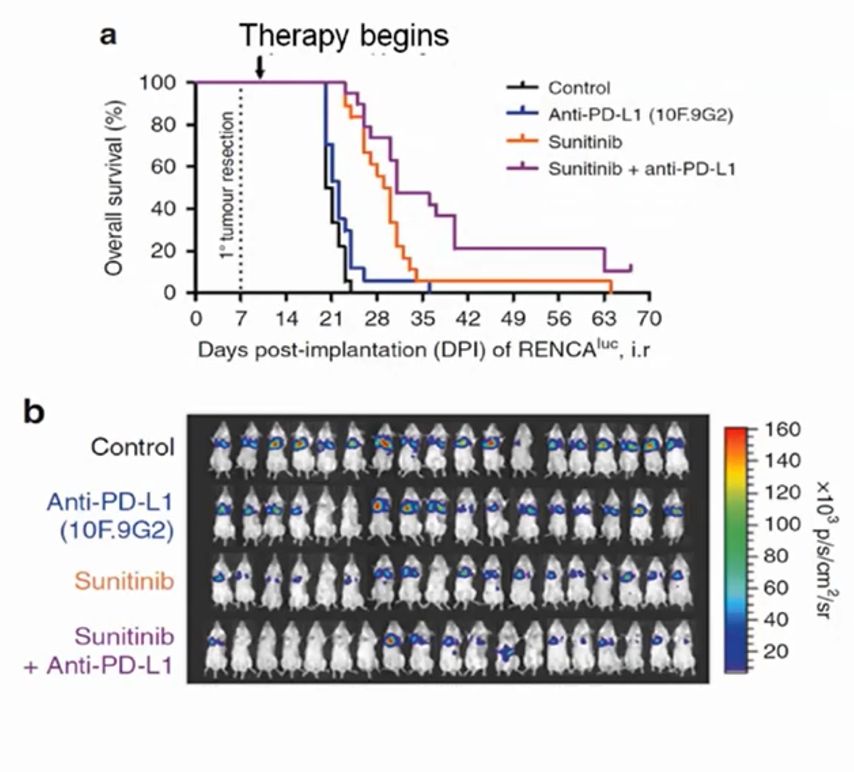 Such an approach has now been adopted in the use of axitinib with avelumab or pembrolizumab in patients with advanced renal cell carcinoma based on benefits seen, compared to sunitinib, in phase III RCTs. More recently, the CheckMate-9ER trial demonstrated a benefit to the combination of cabozantinib and nivolumab, in a similar manner.
Such an approach has now been adopted in the use of axitinib with avelumab or pembrolizumab in patients with advanced renal cell carcinoma based on benefits seen, compared to sunitinib, in phase III RCTs. More recently, the CheckMate-9ER trial demonstrated a benefit to the combination of cabozantinib and nivolumab, in a similar manner.
In summary, Dr. Kerbel emphasized the importance of recapitulating human treatment paradigms in animal models in order to improve the concordance of results between animal and human studies.
Presented by: Robert S. Kerbel, PhD, University of Toronto
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Contact: @WallisCJD on Twitter at the 2020 Société Internationale d'Urologie Virtual Congress (#SIU2020), October 10th - October 11th, 2020


