Athens, Greece (UroToday.com) Dr. R. Jeffrey Karnes gave a presentation overview of the treatment options of patients with biochemical recurrence (BCR) after radical prostatectomy (RP).
He began his talk with some important observations:
- BCR occurs in one-third of patients by 10 years following RP.
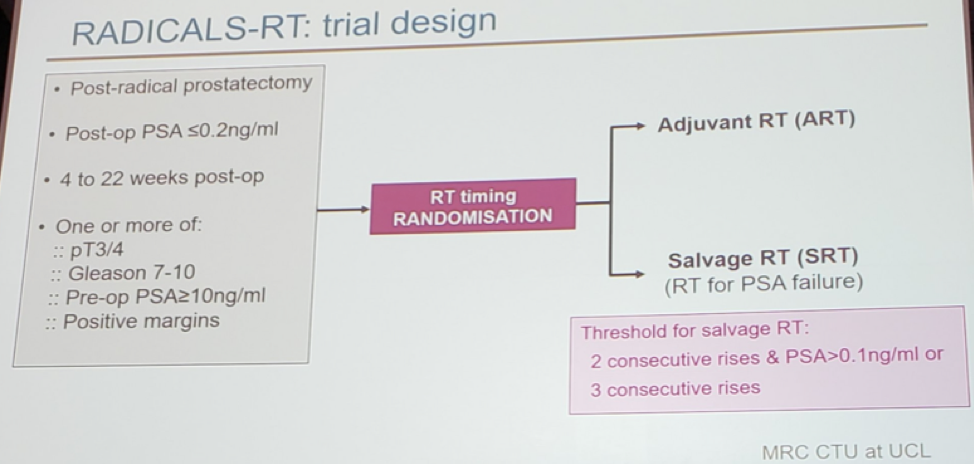
- We now have level one evidence from the RADICALS trial1 showing no advantage of adjuvant radiotherapy (ART) over salvage radiotherapy (SRT), as was recently presented in ESMO 2019, in Barcelona
- It is important that we balance between undertreatment and overtreatment of these patients.
- Therapy in these patients needs to be personalized, with imaging and genomics now becoming part of the workup and treatment
Once BCR occurs, there are several therapeutic options available to us. These include:
- Surveillance
- Imaging
- Initiation of androgen deprivation therapy (ADT)
- Salvage radiotherapy given at ~66Gy +/- ADT
- Metastases directed therapy (MDT) either as radiotherapy or salvage lymph node dissection
- Local therapy (in the form of surgery or ablation)
The role of ADT in this scenario is still not clear with conflicting results2. Early ADT cannot be recommended as the standard of care, and it might only be considered in patients with a PSA doubling time (PSADT) of less than 6-12 months, Gleason score >7 and long-life expectancy. ADT should be offered as part of SRT, but this is not a “one size fits all” treatment. It should be considered only if the postoperative PSA is higher than 0.7 ng/ml and most likely for a duration of 4-6 months only.
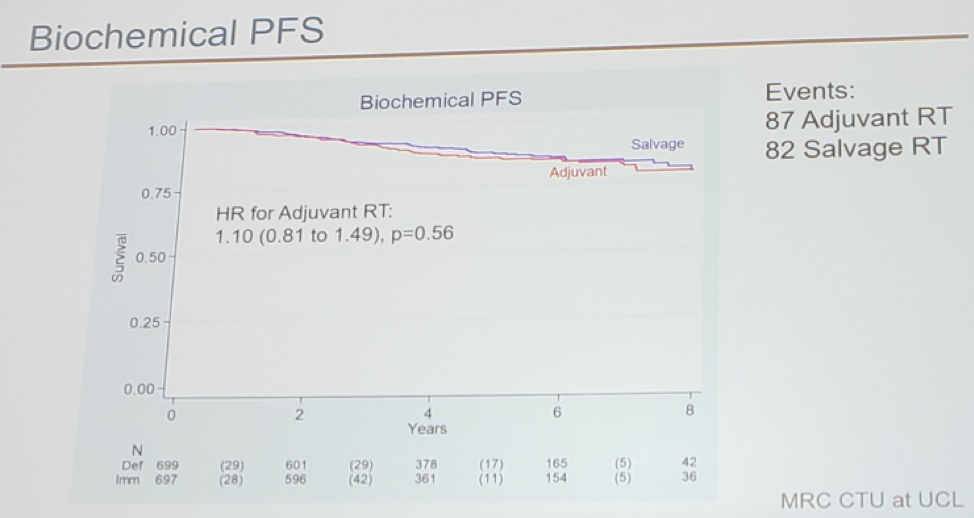
The timing of radiotherapy in these patients has recently been addressed in the RADICALS trial1, presented at ESMO 2019, in Barcelona. This study showed no difference in biochemical progression-free survival (PFS) between adjuvant and salvage radiotherapy (SRT) (figure 1). This will eventually result in SRT becoming the new standard in the next few years. In the ARTISTIC collaboration meta-analysis, incorporating results of the RADICALS, GETUG-AFU 17, and RAVES3 trials, also presented at ESMO 2019, a potential absolute difference of 1% at 5-year (in favor of SRT) was shown.
Figure 1 – RADICALS trial design and results showing no difference in biochemical progression-free survival between adjuvant and salvage radiotherapy
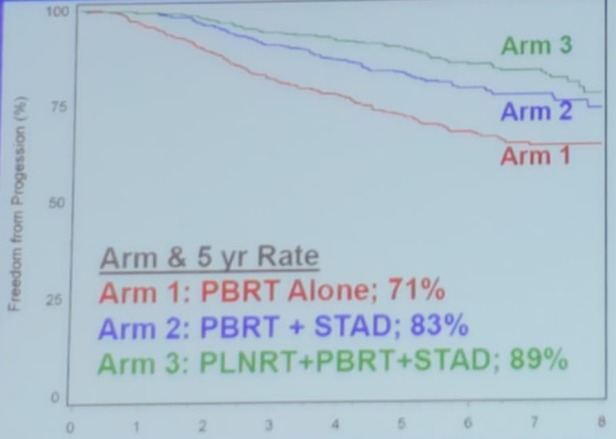
The RTOG 0534 trial assessed short term androgen deprivation therapy without or with pelvic lymph node treatment added to prostate bed only salvage radiotherapy in patients with BCR. This study showed strong evidence supporting pelvic radiotherapy. The number needed to prevent 1 progression in 5 years was 5 (Figure 2).
Figure 2 – RTOG 0534 trial results demonstrating an advantage in patients treated with pelvic lymph node treatment (PLNRT), prostate bed radiotherapy (PBRT) and short-term ADT (STAD):
The role of multiparametric MRI (mpMRI) was discussed next. There is data showing that mpMRI is an independent predictor of SRT outcomes after RP4. In a study assessing 473 patients with SRT, 57% of patients had a mpMRI lesion. The study also showed a median PSA of 0.4 ng/ml for patients without a visible mpMRI lesion and a median PSA of 0.46 ng/ml for patients with a visible mpMRI lesion.
There are nomograms incorporating mpMRI results for early SRT (Table 1). Dr. Karnes state that he personally uses mpMRI for BCR patients before using PET PSMA. According to him, If mpMRI is negative at a PSA level of 0.5 ng/ml, then he will usually wait with PET PSMA until the PSA level reaches 1 ng/ml.
Table 1 – Nomogram incorporating mpMRI results for early SRT:

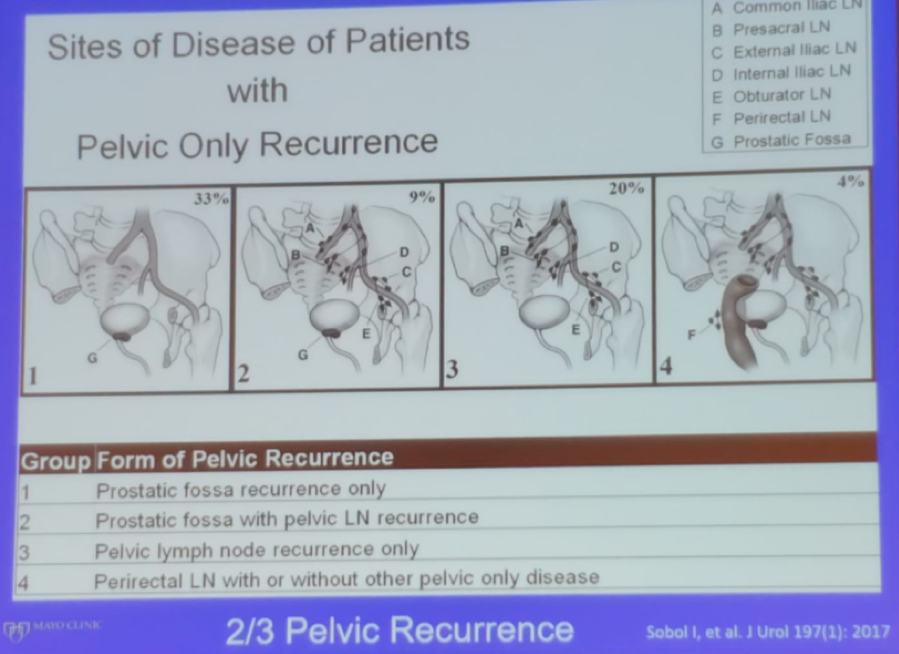
Dr. Karnes moved on to discuss the sites of disease in patients with pelvic only recurrence. He described the classification of pelvic recurrences5 (Figure 3). The sites of recurrence can be successfully identified in the majority of men (>75%) after RP at a median PSA of 2.3 ng/ml with “updated” imaging. The median time from BCR to the identification of disease with the use of advanced PET PSMA imaging is now known to be 15 months and not 8 years, as has been believed before, according to the landmark trial of by Pound et al6. Recurrence confined to the pelvis without other sites of distant metastases accounts for 66% of patients with BCR. These findings will eventually set the stage for MDT, as more data continues to be reported.
Figure 3 – Sites of disease of patients with pelvic only recurrence:
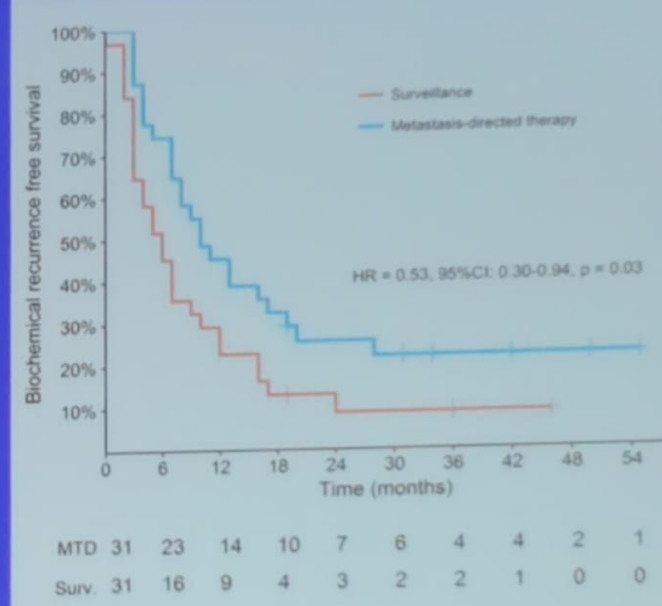
The STOMP trial7 assessed and compared surveillance or MDT for oligometastatic prostate cancer recurrence (study design is shown in figure 4). In this study, the reasons to start ADT were local progression, symptomatic progression or polymetastatic progression7. The study demonstrated that 75% of patients who received MDT had a PSA decline (Figure 5).
Figure 4 – STOMP study design:

Figure 5 – STOMP trial results:
The last topic discussed was the development and validation of a genomic classifier (Decipher) to predict metastases in patients with BCR. The Decipher score confers a continuous risk score giving a probability of metastases at five years. It consists of 22 expressed RNA biomarkers and reflects multiple biologic pathways. For its use, tissue derived from the RP specimen is used. The DECIPHER has been used to identify men with adverse pathology after RP who benefit from adjuvant radiation therapy8. This test has been shown to predict those who benefit from adjuvant radiotherapy and those who benefit from SRT8.
Dr. Karnes concluded his talk stating that genomics and PET PSMA imaging will be incorporated more significantly in the management of patients with BCR in the future, and the standardization of these additions will greatly help treat those patients.
References:
- Pearse M, Fraser-Browne C, Davis ID, et al. A Phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: background and rationale of the Radiotherapy -- Adjuvant Versus Early Salvage (RAVES) trial. BJU international 2014; 113 Suppl 2: 7-12.
- van den Bergh RC, van Casteren NJ, van den Broeck T, et al. Role of Hormonal Treatment in Prostate Cancer Patients with Nonmetastatic Disease Recurrence After Local Curative Treatment: A Systematic Review. Eur Urol 2016; 69(5): 802-20.
- Kneebone A, Fraser-Browne C, Delprado W, et al. A Phase III Multi-Centre Randomised Trial comparing adjuvant versus early salvage Radiotherapy following a Radical Prostatectomy: Results of the TROG 08.03 and ANZUP “RAVES” Trial. International Journal of Radiation Oncology • Biology • Physics 2019; 105(1): S37-S8.
- Sharma V, Nehra A, Colicchia M, et al. Multiparametric Magnetic Resonance Imaging Is an Independent Predictor of Salvage Radiotherapy Outcomes After Radical Prostatectomy. Eur Urol 2018; 73(6): 879-87.
- Sobol I, Zaid HB, Haloi R, et al. Contemporary Mapping of Post-Prostatectomy Prostate Cancer Relapse with (11)C-Choline Positron Emission Tomography and Multiparametric Magnetic Resonance Imaging. The Journal of urology 2017; 197(1): 129-34.
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama 1999; 281(17): 1591-7.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018; 36(5): 446-53.
- Den RB, Yousefi K, Trabulsi EJ, et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015; 33(8): 944-51.
Presented by: R. Jeffrey Karnes, MD, Urologist, Mayo Clinic, Rochester, Minnesota, United States
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New-York, USA @GoldbergHanan at the 39th Congress of the Société Internationale d'Urologie, SIU 2019, #SIUWorld #SIU2019, October 17-20, 2019, Athens, Greece


