When thinking of the ideal biomarkers in the pre-biopsy setting, it should increase the probability of achieving a positive biopsy. Unfortunately, the positive predictive value of the most used biomarker to date for prostate cancer (PSA), is very low (25-40%) when it is in the range of 4-10 ng/ml.1 Approximately 65-75% and 10-35% of initial and repeat biopsies in the PSA range of 4-10 ng/ml are negative.2 Additionally, the ideal biomarker will reduce overdetection and the number of unnecessary biopsies, as biopsies have significant adverse events (infection, bleeding), and are associated with pain.3
Prostate cancer biomarkers in the pre-biopsy setting can be divided to those used to ascertain who to biopsy (PHI, 4K score, Select MDX, MiPS, ExoDx prostate Intelliscore), and those used to ascertain when to re-biopsy (PCA3, and ConfirmMDx). According to the European Association of Urology (EAU) guidelines, to avoid unnecessary biopsies, it is recommended to use risk calculators and additional biomarkers, or imaging.
Next, Dr. Tilki gave a summary of the most important available biomarkers. She began with the biomarkers that are supposed to help decide which patient should be biopsied. The first ones discussed were the PHI and the 4K score. The PHI combines three PSA sub-forms (total PSA, free PSA, and [-2]proPSA) into a single score. It is indicated in men with a PSA of between 2-10 ng/ml and normal digital rectal examination (DRE). The PHI score has been shown to correlate with the percentage of a positive biopsy, as seen in figure 1.
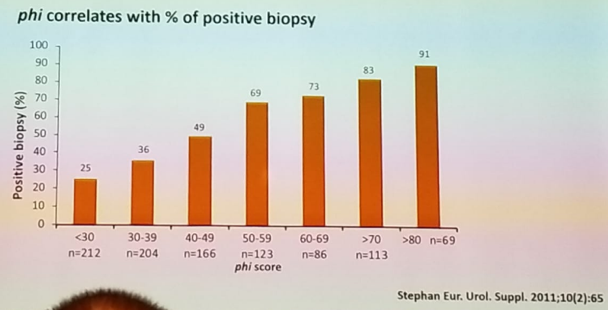
Figure 1- PHI score is correlated to the percentage of positive biopsy
The 4K score combines four prostate-specific kallikrein assay results (total PSA, free PSA, intact PSA, and hk2) with age and prior biopsy status. The 4K score gives the percentage risk of having aggressive prostate cancer, and it has been shown to predict the probability of distant metastasis within 20 years, as seen in figure 2.
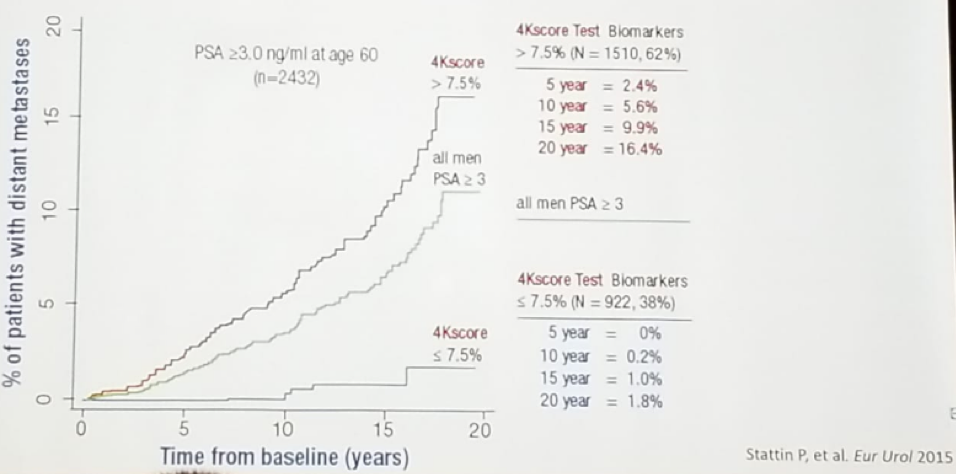
Figure 2 – 4K score prediction of metastasis within 20 years
In a comparison of 4k to PHI, they were shown to similarly improve discrimination when predicting prostate cancer and high-grade prostate cancer. Both are simple blood tests that can reduce the number of unnecessary biopsies compared with screening using PSA only.4 Usage of the 4K score in a hypothetical cohort of 100000 men suspected of having prostate cancer, can result in an average net savings of 169 million dollars (1694 USD per patient), during the first year after the first urologist visit.5
The next biomarker discussed was the SelectMDx, which is a non-invasive urine assay to improve patient selection for initial biopsy. It is a two-gene risk score combining HOXC6 and DLX1 mRNA expression levels with clinical risk factors, including PSA density, DRE, PSA, age, history of prostate biopsy, and family history. This biomarker can detect high-grade clinically significant prostate cancer. It has an area under the curve (AUC) of 0.89 for detecting clinically significant cancer, and it is highly correlated with Gleason score as well, with a negative predictive value of 98% for aggressive cancer. Various cost-effectiveness studies of SelectMDx have been done, demonstrating its ability to significantly save money.
The next biomarker discussed was the MI-prostate score. This score consists of PCA3 mRNA urine test, total PSA in the blood, and TMPRSS2-ERG mRNA in the urine. It can predict the presence of overall and high-grade prostate cancer on initial and repeat biopsy. (6) It has been shown to have an AUC of 0.772 for detection of prostate cancer.7
ExoDX prostate (Intelliscore) was the next biomarker discussed. This urine biomarker utilized exosomes, which are small, double-lipid membrane vesicles that are secreted from cells. This is a urinary exosome gene expression assay which predicts the presence of high-grade prostate cancer for men older than 50 with a PSA of 2-10 ng/ml, presenting for an initial biopsy. It gives a score between 0-100 by combining the relative weighted expressions of three gene signatures. A score>15.6 is associated with an increased likelihood of high-grade prostate cancer on a subsequent biopsy. It has an AUC of 0.77 and has been validated in a large multicenter study.8
Dr. Tilki moved on to discuss the two biomarkers used to ascertain when to biopsy. These are the PCA3 and ConfirmMDx. The PCA3 urine test is analyzed by RT-PCR and is an assay commercially used today for aiding the diagnosis of prostate cancer. This has been used especially in patients with a previous negative prostate biopsy. PCA3 has been recommended in for men at a higher risk of prostate cancer, with previous negative needle biopsy.
The ConfirmMDx detects a field effect or halo associated with the presence of cancer at the DNA level. It detects epigenetic changes and is performed on the residual tissue from previous negative biopsies (up to 30 months old). It helps to distinguish patients who have a true-negative biopsy from those who may have occult cancer. The test helps find men who may benefit from a mpMRI or another biopsy and early detection. It has 90% and 96% negative predictive value for the detection of any cancer and significant prostate cancer, respectively. In a cost-effectiveness analysis, it has been shown to reduce 1106 unnecessary biopsies for a health plan with 1 million members, with more than 588 USD saved per patient, and more than 530000 USD saved annually per 1 million members.9
Dr. Tilki summarized her talk, showing a nice algorithm which helps to choose the right genomic biomarker in the appropriate phase (Figure 3). There is currently a large Dutch multicenter prospective study which is comparing mpMRI, biomarkers and risk calculators in approximately 600 patients with a PSA of more than three ng/ml. The recruitment has been completed, and the results are expected at the end of this year.
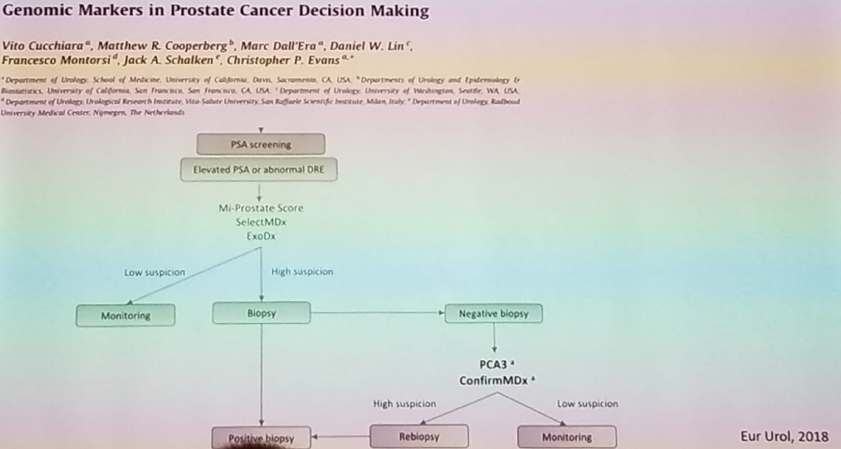
Figure 3 – Algorithm on the appropriate genomic biomarker to use:
In summary, pre-biopsy markers can improve prostate cancer detection and reduce overdiagnosis. Results from economic evaluations have shown cost-effectiveness for a 4K score, SelectMDx, and ConfirmMDx. To date, the optimization of biomarker uses in the pre-biopsy setting, and their combination with novel imaging remains unclear.
References:
1. Hendricks RJ et al. Pros Cancer Prostatic Dis. 2016
2. Heidenreich A et al. Eur Urol 2008
3. Loeb S et al. Eur Urol 2013
4. Nordstrom et al. Eur Urol 2015
5. Voigt et al. Rev Urol 2017
6. Sanda MG et al. JAMA Oncol 2017
7. Tomlins SA et al. Eur Urol 2016
8. McKiernan et al. JAMA Oncol 2016
9. Aubry W et al. American Health Drug and Benefits 2013
Presented by: Derya Tilki, MD, Martini-Klinic, Hamburg, Germany
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre Twitter: @GoldbergHanan at the 38th Congress of the Society of International Urology - October 4- 7, 2018 - Seoul, South Korea


