According to Dr. Sanches-Salas, focal therapy can be recommended in D’amico low and intermediate risk disease. In clinically localized cancer, with good life expectancy and a single favorable lesion, focal therapy is an appropriate strategy for Gleason Cancer 3+4. Men with intermediate risk cancer and a PSA of less than 10 ng/ml should be considered candidates for focal therapy.
Screening leads to an increase in prostate cancer diagnosis. However, most patients are diagnosed with a non-clinically significant disease, where radical treatment is not appropriate. Therefore, less aggressive options have arisen, enabling us to personalize the treatment to control the disease while avoiding toxicity. Although focal therapy is not the standard treatment for men with organ-confined disease, it is the therapeutic approach with the most important future potential1 However, to date, focal therapy is still an experimental option for prostate cancer, and long-term follow-up data is still lacking. Focal therapy treats the index lesion, which is the largest or most aggressive lesion. The index lesion defines the tumor volume, Gleason score, and pathological stage in the multifocal disease. Lastly, the index lesion drives the cancer progression.
The multiparametric MRI is an important tool for focal therapy. The PROMIS trial demonstrated the mpMRI to have a sensitivity of 93% for clinically significant cancer.2 Additional studies demonstrated a negative predictive value of 88.1% for mpMRI3 and a mpMRI in combination with comprehensive sampling was able to achieve a negative predictive value of 90-95%. Lastly, the PRECISION trial demonstrated that mpMRI before biopsy and mpMRI targeted biopsy is superior to standard transrectal ultrasonography-guided biopsy.4 The limitations of the mpMRI is in defining the actual size and borders of the significant lesions. Therefore, any ablation performed should account for a reasonable security margin.
The ideal patient for focal therapy is the patient with intermediate-risk disease with a Gleason of 3+4. Low-risk prostate cancer without indication for active surveillance could also be considered for partial gland focal therapy ablation.5 Eligibility criteria for focal therapy should be based on the National Comprehensive Cancer Network (NCCN) intermediate risk definition and recent consensus guidelines, which state that 30-38% of patients could be indicated for a quadrant of Hemi ablation according to the mpMRI and fusion biopsy selection. When assessing people with lower risk or favorable intermediate-risk disease, and then referring them to a mpMRI + fusion biopsy, it is known that 1/3 of them will be eligible for focal therapy.
A patient designated to undergo focal therapy should have a mpMRI and undergo biomarker and multigene expression tests. There are many possible focal therapy therapeutic options, including HIFU, cryotherapy, VTP, electroporation, and brachytherapy. The follow-up protocol for the focal therapy patient includes routine PSA measurement, mpMRI, and systemic biopsy. The reported complication rate is 37.6% in HIFU6 and the majority of these complications occur within the first 30 days of therapy. The outcomes of focal therapy demonstrate a recurrence rate of between (0-64%), depending on the series. In a randomized prospective controlled trial comparing vascular targeted photodynamic therapy (VTP) vs. active surveillance.7 Over a 24 months follow-up period, VTP demonstrated a median time to progression of 28.3 months vs. 14.1 months in the active surveillance group. When comparing active surveillance to TOOKAD therapy, after 36 months of follow-up the rate of needing radical treatment was 24% and 53% in the TOOKAD and active surveillance treatment arms, respectively, as shown in Figure 1.
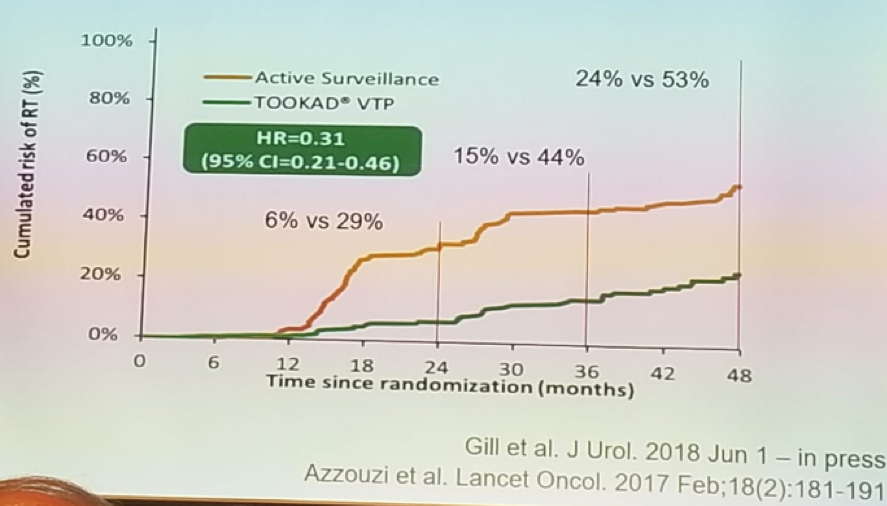
Figure 1 – The need for radical treatment in focal therapy and active surveillance:
When comparing focal therapy to radical therapy, biochemical recurrence had occurred in 6.1% of radical therapy patients and 9% in focal therapy patients. Focal therapy failure had occurred in 28.8% of cases.8 However, continence and impotence rates are much better in the focal therapy patients.8 Lastly, metastasis-free survival for focal therapy has been reported to be 94.6% for grade group 1, 78.1% for grade group 2, and 67.4% for grade group 3.9
Dr. Rafael Sanchez-Salas summarized and concluded his talk stating that focal therapy consists of ablation of the index lesion (plus margin) in men with low or intermediate risk of clinically significant disease. Definitive data on long-term cancer control are still lacking. Focal therapy represents a promising alternative to treat low-risk prostate cancer or favorable intermediate-risk Gleason 3+4 prostate cancer. With accurate localization of the index lesion, focal therapy can provide uncompromised oncologic outcome with significantly less comorbidity, and with excellent functional preservation.
References:
1. Ramsay et al. 2015, Health Technol Assess.
2. Ahmed HU et al. The Lancet 2017
3. Edison et al, Urol Clin. North. Am. 2017
4. Kasivisvanathan V et al. NEJM 2018
5. Nassiri et al. J URol 2018
6. Bakavicius et al. AUA 2018
7. Azzouzi et al. Lancet Oncology 2017
8. Garcis-Barreras et al. J Urol 2018
9. Tourinho-Barbosa et al. 2018
Presented by: Rafael Sanchez-Salas, MD
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre Twitter: @GoldbergHanan at the 38th Congress of the Society of International Urology - October 4- 7, 2018 - Seoul, South Korea
Further Related Content:
Management of Grade Group 2 Prostate Cancer, Surveillance
Management of Grade Group 2 Prostate Cancer, Radical Therapy


