(UroToday.com) The 2024 Southeastern Section of the AUA (SESAUA) annual meeting featured a prostate cancer session and a presentation by Dr. Alexander Chehrazi-Raffle discussing the time interval between Radium-223 therapy and 177Lu-PSMA treatment for mCRPC and outcomes in the RALU study. Radium-223 improves overall survival and quality of life with an acceptable profile in men with bone-predominant mCRPC.1 Additionally, 177Lu-PSMA is a beta-particle emitting therapy that has shown an acceptable safety profile and prolonged overall survival in patients with PSMA-positive mCRPC, previously treated with androgen receptor pathway inhibition and taxane-based chemotherapy.2 Data from a small retrospective study of patients with mCRPC who initiated 177Lu-PSMA therapy within 8 weeks of receiving Radium-223 suggested that the use of both radionuclides within a short time frame is feasible.3 The observational, retrospective study, RALU, investigated the safety and clinical outcomes of sequential Radium-223/177Lu-PSMA therapy in patients with mCRPC. This analysis evaluated the association of time between Radium-223 and 177Lu-PSMA treatments and safety and overall survival outcomes following 177Lu-PSMA.
Data were collected from 2021–2022 in German nuclear medicine centers for all patients receiving 177Lu-PSMA after Radium-223 therapy (n = 132). Patients were stratified into two groups depending on time interval between last Radium-223 dose to first 177Lu-PSMA dose as <6 months (Group 1) or ≥6 months (Group 2). The trial design for RALU is as follows:
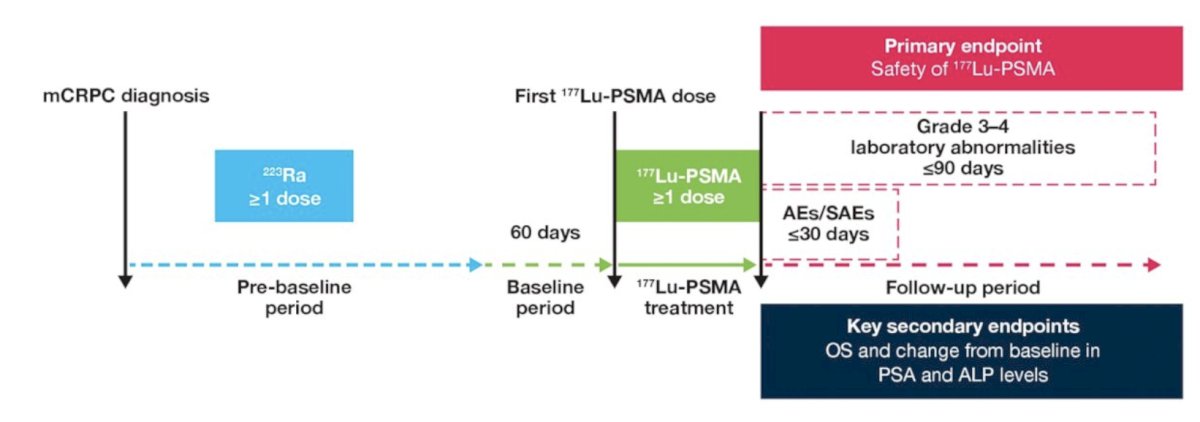
There were 42 patients that received 177Lu-PSMA <6 months after Radium-223 (Group 1) and 90 patients received 177Lu-PSMA ≥6 months after Radium-223 (Group 2). Baseline characteristics prior to 177Lu-PSMA therapy in <6 months and ≥6 months groups were, respectively: median age, 72 and 74 years; Eastern Cooperative Oncology Group performance status (ECOG PS) 1, 57% and 63%; ECOG PS 2, 43% and 37%; median PSA, 366 and 268 ng/mL; median alkaline phosphatase, 133 and 149 U/L. In all, 40% and 64% of patients, respectively, received ≥4 life-prolonging therapies before starting 177Lu-PSMA.
All patients had prior Radium-223; 57% and 77%, respectively, received six Radium-223 injections. Prior to 177Lu-PSMA, 24% and 29% of patients had visceral metastases, and 45% and 52% of patients received ≥4 177Lu-PSMA cycles. From 177Lu-PSMA start to ≤30 days post last dose, 71% and 82% of patients, respectively, had treatment-emergent adverse events of any grade. The most common were fatigue (12% vs 7%), nausea (12% vs 8%), and dry mouth (7% vs 18%).
There were 36% and 24% of patients that had Grade 3–4 treatment-emergent adverse events, and excluding laboratory abnormalities, osteonecrosis of the jaw was the most frequent Grade 3–4 treatment-emergent adverse events (5% vs 2%).
Median overall survival from the start of 177Lu-PSMA was 12.0 months (95% CI: 8.8–19.9) and 13.2 months (95% CI: 10.0–15.9), respectively. During 177Lu-PSMA therapy, PSA response ≥50% occurred in 53% and 39%, and alkaline phosphatase response ≥30% in 28% and 14% of patients, respectively.
Dr. Chehrazi-Raffle concluded his presentation by discussing the time interval between Radium-223 therapy and 177Lu-PSMA treatment for mCRPC and outcomes in the RALU study with the following conclusions:
- Overall survival outcomes from the start of 177Lu-PSMA therapy were similar in men who received Radium-223 within 6 months or 6 months or more prior to initiating 177Lu-PSMA
- Incidences of Grade 3-4 hematological laboratory abnormalities were also similar in the two groups
- Giving 177Lu-PSMA within 6 months of completing Radium-223 therapy was clinically feasible and well tolerated
Presented by: Alexander Chehrazi-Raffle, MD, City of Hope Comprehensive Cancer Center, Duarte, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Southeastern Section of the American Urological Association (SESAUA) Annual Meeting, Austin, TX, Wed, Mar 20 – Sat, Mar 23, 2024.
References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Baumgarten J, Groener D, Ngoc CN, et al. Safety and efficacy of 177Lutetium-PSMA-617 Radioligand Therapy Shortly after Failing 223Radium-Dichloride. Cancers (Basel). 2022 Jan 22;14(3):557.


