(UroToday.com) The 2023 SESAUA annual meeting included a State-of-the-Art lecture presentation by Dr. Kristen Scarpato discussing what we should know about prostate biopsies in 2023. Dr. Scarpato started her presentation by highlighting that the goals of prostate biopsy are several, including (i) cancer detection (specifically clinically significant prostate cancer), (ii) patient safety (reducing infection, over-detection, and over-treatment), and (iii) patient experience.
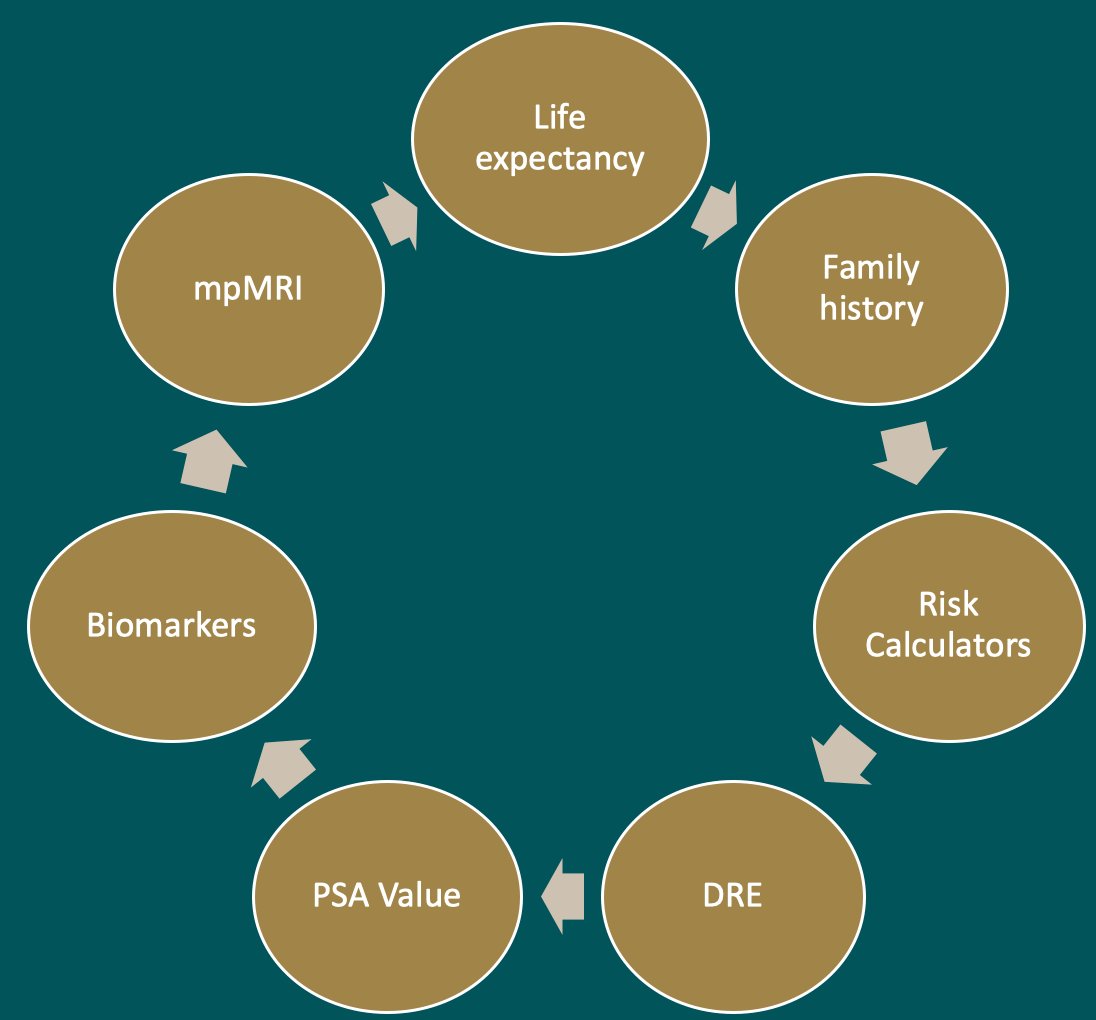
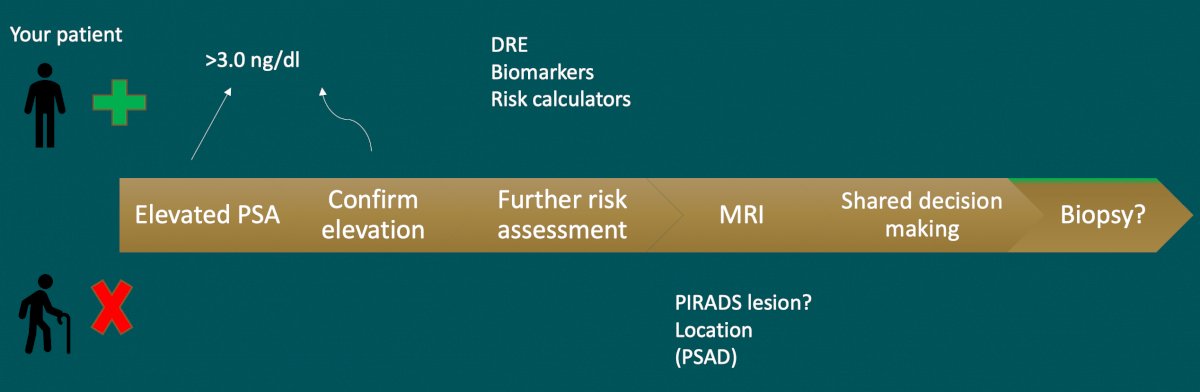
The first major question is: Who should we biopsy? Risk assessment includes many factors including life expectancy, family history, risk calculators, digital rectal examination, PSA values, biomarkers, and multiparametric MRI results:
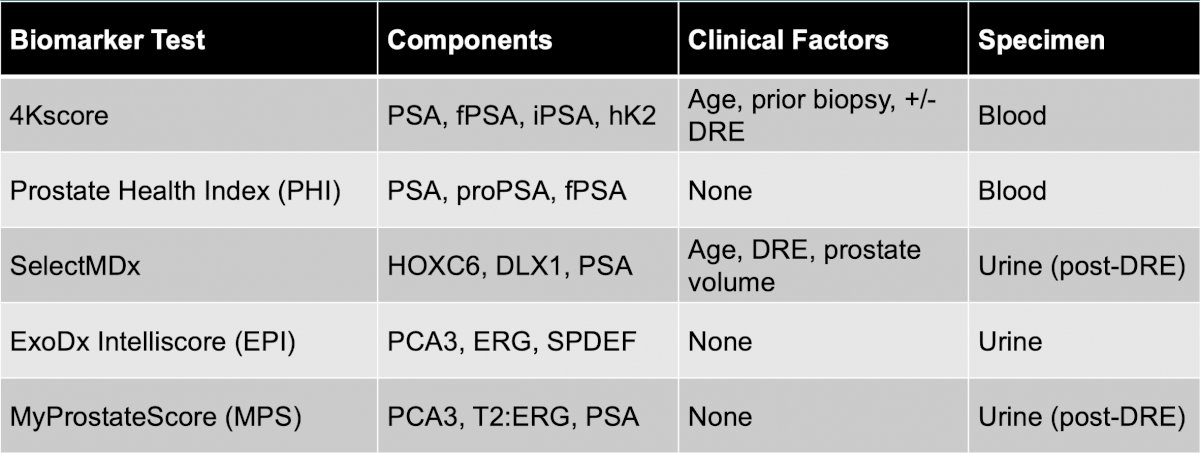
With regards to prostate cancer biomarkers, Dr. Scarpato notes that there are several available, including 4Kscore, Prostate Health Index (PHI), SelectMDx, ExoDx Intelliscore (EPI), and MyProstateScore (MPS):
One of the biggest challenges is how we manage a negative multiparametric MRI, given that what we miss when a biopsy is omitted in the setting of a negative MRI is potentially clinically significant prostate cancer. The important metric here is negative predictive value, ie the likelihood that a negative MRI means no clinically significant prostate cancer. Two meta-analyses have assessed negative predictive value in the setting of a negative MRI, including Sathianathen et al.1 reporting a negative predictive value of 90.8% for >= GG2 disease and 97.1% for >= GG3 disease. Additionally, Moldavan et al.2 reported a negative predictive value of 88.1% for >=GG2 disease.
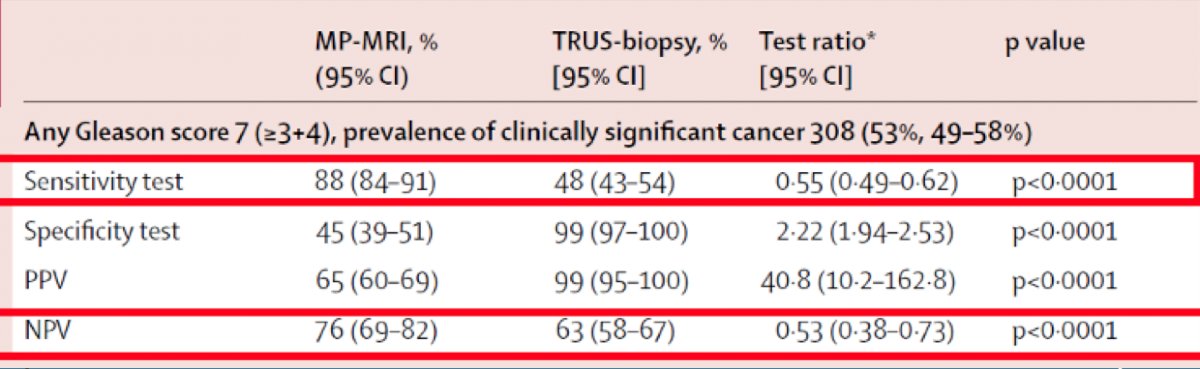
Dr. Scarpato then discussed two key trials, the first of which is the PROMIS3 trial. PROMIS was a multi-center, paired-cohort study to assess the diagnostic accuracy of mpMRI and transrectal ultrasound guided biopsy against a gold-standard reference template mapping biopsy. Men were included (n=576) if they had a PSA <15 ng/ml and no history of previous biopsy. On mapping biopsy, 71% of men had cancer, including 40% with clinically significant prostate cancer (Gleason score ≥4+3 or maximum cancer length ≥6 mm). For clinically significant disease, mpMRI was more sensitive (93%) than transrectal ultrasound guided-biopsy (48%), albeit less specific (41% for mpMRI; 96% for transrectal ultrasound guided-biopsy). The sensitivity, specificity, positive predictive value, and negative predictive value for clinically significant prostate cancer is as follows:
Based on these data, a triage mpMRI would allow 25% of men to safely avoid a prostate biopsy, while at the same time reducing detection of clinically insignificant prostate cancer. Importantly, secondary to the poor specificity and positive predictive value, this study does not suggest that mpMRI should replace prostate biopsy and that men with suspicious lesions should still have histologic confirmation of prostate cancer.
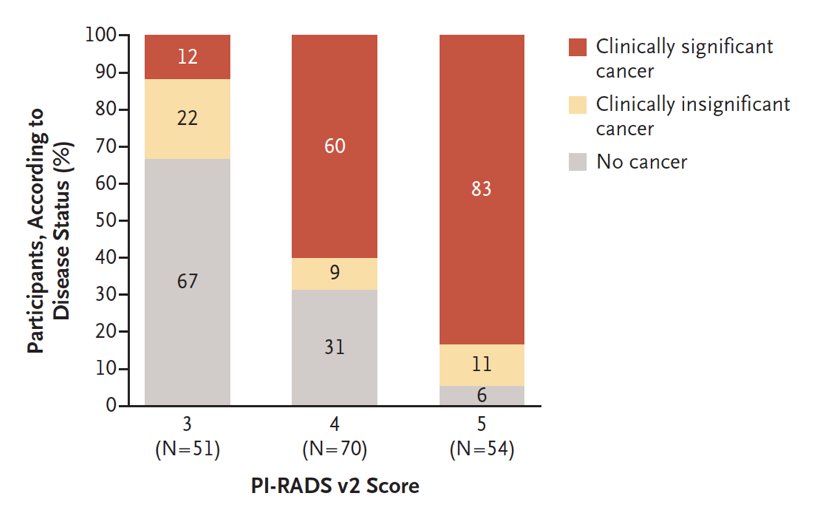
The second key trial is the PRECISION trial,4 which assigned 500 men with a clinical suspicion of prostate cancer who had not previously undergone a prostate biopsy to undergo MRI with or without a targeted biopsy vs standard transrectal ultrasound guided-guided biopsy. Men in the MRI group underwent a targeted biopsy if there was a suspicion of prostate cancer on imaging and did not undergo a biopsy if the MRI was negative. The primary outcome of this randomized clinical trial was a diagnosis of clinically significant prostate cancer. In the MRI-targeted biopsy group, 28% had a negative MRI and thus no biopsy. Among men undergoing targeted biopsy, 38% had clinically significant cancer, compared to 26% in the transrectal ultrasound guided-guided biopsy group (p=0.005). Furthermore, fewer men in the MRI-targeted biopsy group had clinically insignificant prostate cancer compared to the transrectal ultrasound guided-guided biopsy group. As follows is the biopsy breakdown by PI-RADS score:
Based on the aforementioned data, Dr. Scarpato summarized that a negative MRI + no prostate biopsy leads to a ~0-12% risk of missing clinically significant prostate cancer. According to the NCCN guidelines, an mpMRI is strongly recommended in the setting of clinical suspicion of prostate cancer. Additionally, the EAU guidelines make the following recommendations:
- For biopsy naïve patients: perform an MRI before prostate biopsy (Strength rating: Strong); when an MRI is negative (ie. PI-RADS <=2) and clinical suspicion of prostate cancer is low (ie. PSA density < 0.15 ng/mL), omit biopsy based on shared decision making with the patient (Strength rating: Weak)
- For patients with a prior negative biopsy: perform MRI before a prostate biopsy (Strength rating: Strong)
Dr. Scarpato emphasized that shared decision making is key in these clinical situations. A collaborative process improves the quality of the medical decision making, as there is no single best management approach. These decisions are based on the patient’s values, individual risks, and comorbidities, taking into account the patient’s immediate and long-term risks. As follows is a simple schema for informed decision making regarding prostate cancer screening:5

The following is a summary of the clinical work flow for patients with an elevated PSA in Dr. Scarpato’s practice:
The second part of Dr. Scarpato’s talk focused on how we should biopsy patients. She notes that 95% of prostate biopsies are performed with standard transrectal ultrasound guidance, however, although transrectal ultrasound guidance shows the contour of the prostate, lesions are not well visualized, sampling is random, and generally, there is under sampling of the prostate. Furthermore, anterior and apical lesions may be missed, and tumors may be understaged, undergraded, and missed in 1/3 of cases. An additional consideration with transrectal ultrasound guidance is potential infectious complications, which appear to be increasing. This may include 2-7% of patients having a urinary tract infection, epididymitis, orchitis, prostatitis, and in severe situations having sepsis. Furthermore, there is increasing fluoroquinolone resistance in the community, including 25% of men undergoing prostate biopsy. As such, improvements to transrectal ultrasound guidance biopsy may include enhanced antibiotic prophylaxis, assessing the local antibiogram, and rectal swabs. Additionally, perhaps a different approach, such as transperineal biopsy is warranted:
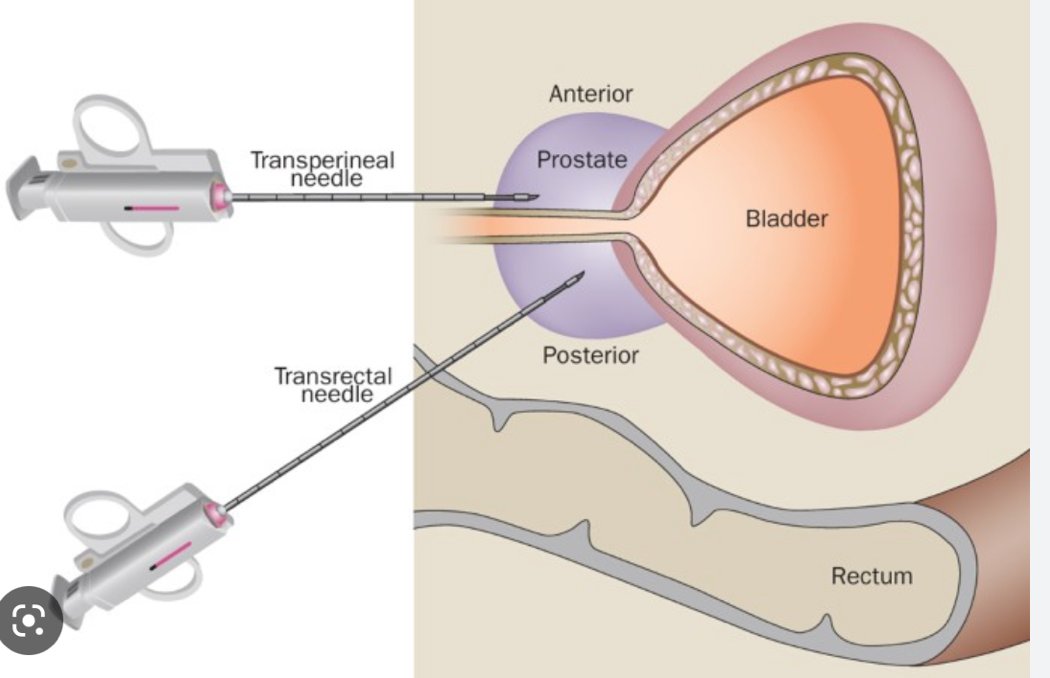
Dr. Scarpato summarized that the transperineal biopsy literature suggests there is no difference to transrectal ultrasound guided biopsy for clinically significant prostate cancer detection rates, there is variable use of antibiotics in these trials/studies, most studies report zero admissions for infectious complications, with an overall complication rate <0.01%. The EAU guidelines provide the following prostate biopsy workflow to reduce infectious complications:
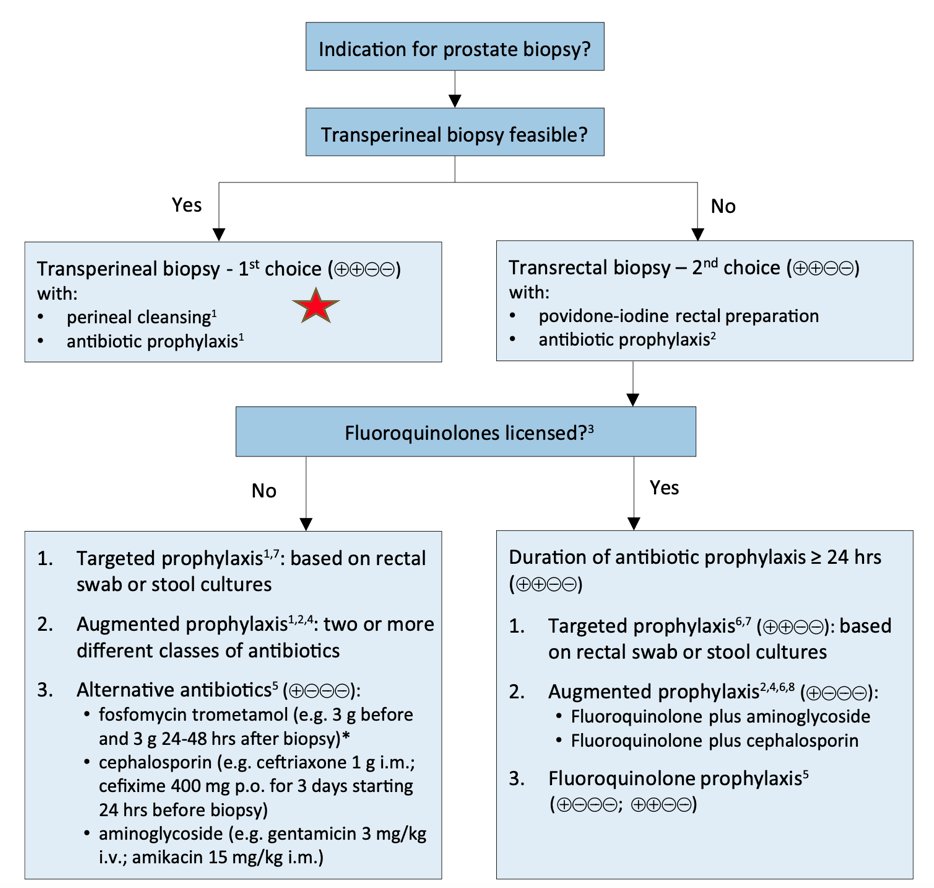
Dr. Scarpato then highlighted several considerations for the transperineal biopsy approach:
- Cost: for the ultrasound machine, probes, stirrups, use of an angiocath versus needle guide
- Learning curve: ~10 procedures
- Local anesthesia: the biggest challenge to this approach
- Procedure time: <10 minutes
- Outcomes: cancer detection (better) and complications (few)
Dr. Scarpato then shifted focus to discuss the importance of multiparametric MRI, which should be used as a screening tool and is useful at multiple points in the evaluation of prostate cancer. In addition to providing excellent anatomic and functional detail, the PI-RADS v2.1 simplifies acquisition, interpretation, and reporting. Importantly, based on multiparametric MRI findings, lesions can be targeted for biopsy via an in-bore technique, cognitive fusion, or MRI-US fusion technique.
In 2015, Siddiqui and colleagues published a trial comparing MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy.6 Among 1,003 men targeted MR/ultrasound fusion biopsy diagnosed 461 prostate cancer cases compared to 469 cases for standard biopsy. Targeted biopsy diagnosed 30% more high-risk cancers vs standard biopsy (173 vs 122 cases, p < 0.001) and 17% fewer low-risk cancers (213 vs 258 cases, p < 0.001). When standard biopsy cores were combined with the targeted approach, an additional 103 cases (22%) of mostly low-risk prostate cancer were diagnosed (83% low risk, 12% intermediate risk, and 5% high risk). In 2021, Klotz et al.7 assessed whether MRI with only targeted biopsy was noninferior to systematic transrectal ultrasound guided biopsies in the detection of GG 2 or greater prostate cancer. Among 453 patients, a lesion with a PI-RADS score of 3 or greater was detected in 138 of 221 men (62.4%) who underwent MRI. Importantly 83 of 221 men who underwent MRI-targeted biopsy (37%) had a negative MRI result and avoided biopsy. Cancers GG2 and greater were identified in 67 of 225 men (30%) who underwent transrectal ultrasound guided biopsy, compared to 79 of 227 (35%) allocated to MRI-targeted biopsy, thus this approach was deemed non-inferior. Dr. Scarpato concluded that MRI targeting leads to better detection of GG >2 disease in the initial and repeat biopsy setting and lower detection of GG1 prostate cancer. Based on the EAU guidelines for men with a prior negative biopsy, targeted biopsies only can be performed when an MRI is positive (PI-RADS >=3) (Strength rating: Weak), and when MRI is negative (PI-RADS <=2), and clinical suspicion of prostate cancer is high, we should perform a systematic biopsy based on shared-decision making with the patient (Strength rating: Strong).
Dr. Scarpato then highlighted the 2022 NEJM GÖTEBORG-2 trial8 assessing prostate cancer screening with PSA and MRI followed by targeted biopsy only. Among 37,887 men 50-60 years of age invited for the screening trial, 17,980 (47%) participated in the trial. There were 66 of the 11,986 participants in the experimental group (0.6%) were diagnosed with clinically insignificant prostate cancer, compared with 72 of 5994 participants (1.2%) in the reference group [difference of -0.7 percentage points (95% CI -1.0 to -0.4; relative risk, 0.46; 95% CI, 0.33 to 0.64)]. Additionally, the relative risk of clinically significant prostate cancer in the experimental group as compared with the reference group was 0.81 (95% CI, 0.60 to 1.1).
Dr. Scarpato summarized MRI for prostate biopsy with the following points:
- Pros:
- Directed biopsies: transrectal ultrasound guided or transperineal
- Improved detection of clinically significant prostate cancer
- Perhaps the ability to limit the number of biopsies
- Perhaps the ability to avoid a prostate biopsy
- Cons:
- Two visits
- Patient factors (size, anxiety)
- Expense
- Dependence on radiology expertise
- Potential for imprecision in targeting
Dr. Scarpato concluded her presentation by discussing the use of multiparametric MRI in active surveillance. Importantly, patients on active surveillance should not avoid a biopsy if the MRI remains unchanged, given that MRI has low sensitivity to predict progression, there is poor association between MRI progression and pathologic progression, and there is inadequate specificity to rule out high-grade disease when the MRI is negative. However, MRI with targeted biopsy prior to the initiation of active surveillance decreases the chance of discontinuing active surveillance at 2 years, and MRI-US fusion detects significantly more grade progression compared to systematic biopsy at 2 years.
Dr. Scarpato concluded her presentation by discussing what we should know about prostate biopsies in 2023 with the following take-home messages:
- Choose the right patient
- Imaging:
- MRI remains an important tool in prostate cancer detection
- Use before a prostate biopsy, and consider omission of a biopsy if the MRI is negative
- Approach:
- Transrectal ultrasound guided and transperineal biopsy each have their pros and cons
- MRI targeted biopsies should be utilized, and targeted only biopsies may be on the horizon
- Emerging transperineal data may indicate an advantage
Presented by: Kristen R. Scarpato, MD, PhD, Vanderbilt University, Nashville, TN
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Southeastern Section of the American Urological Association (SESAUA) Annual Meeting, Amelia Island, FL, Wed, Mar 15 – Sat, Mar 18, 2023.
References:
- Sathianathen NJ, Omer A, Harriss E, et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the Prostate Imaging Reporting and Data System Era: A systematic review and meta-analysis. Eur Urol. 2020 Sep;78(3):402-414.
- Moldovan PC, Van den Broeck T, Sylvester R, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines panel. Eur Urol. 2017 Aug;72(2):250-266.
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017;389(10071):815-822.
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med 2018;378(19):1767-1777.
- Vickers AJ, Edwards K, Cooperberg MR, et al. A simple schema for informed decision making about prostate screening. Ann Intern Med. 2014;161:441-442.
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015 Jan 27;313(4): 390-397.
- Klotz L, Chin J, Black PC, et al. Comparison of multiparametric magnetic resonance imaging-targeted biopsy with systematic transrectal ultrasonography biopsy for biopsy-naïve men at risk for prostate cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2021 Apr 1;7(4):534-542.
- Hugosson J, Mansson M, Wallstrom J, et al. Prostate cancer screening with PSA and MRI followed by targeted biopsy only. N Engl J Med 2022 Dec 8;387(23):2126-2137.


