(UroToday.com) At San Raffaele University Hospital, Prof. Alberto Briganti opened the first session of talks entitled “ Key advances in GU tumors: what have we learned in the last 10 years” of the 1st edition of San Raffaele Urologic Oncology Retreat Meeting “Prostate Cancer (PCa) is one of the fields in which we had the major advancements over the last 15 years, but still many questions remain unanswered” said Briganti.
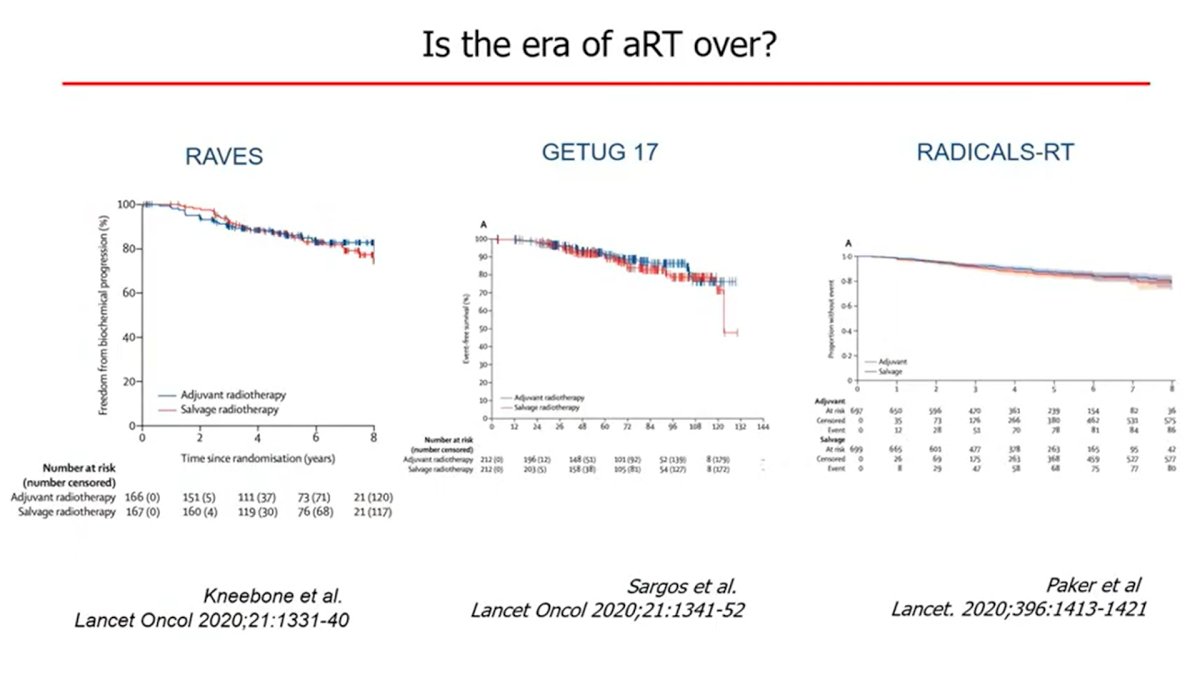
One of the main topics of the last years has been the management of localized high-risk PCa. Prof. Briganti showed the high level of evidence coming from prospective randomized trials demonstrating the benefits of RT in combination with long-term ADT, he continued mentioning the results presented recently by the STAMPEDE group on the benefit of Abiraterone + ADT + RT in the very high-risk localized PCa setting. About surgery, we tested everything, neoadjuvant chemo, neoadjuvant ADT, neoadjuvant novel agents, but there were disappointing results” said Briganti. He continued his talk by presenting the overlapping of the PFS curves about three randomized trials (RAVES, GETUG 17, and RADICALS-RT; fig.1) comparing adjuvant RT vs early salvage RT after radical prostatectomy. Briganti continued showing patients’ characteristics, underlying that only 1in 5 patients had aggressive features. Therefore, these results do not apply to all patients, especially those with aggressive diseases.
In the setting of metastatic PCa we assisted a “huge revolution. 10 years ago, there was mainly ADT alone or together with bicalutamide. But in the last years, things changed significantly”, stated Briganti. Then he showed the state of the Art for metastatic Prostate cancer and the different therapeutic options available both in mHSPC and mCRPC ranging from ADT+ Docetaxel, ADT+ NHA, and the emerging data on triplet therapy association, still with pending approval in several countries.
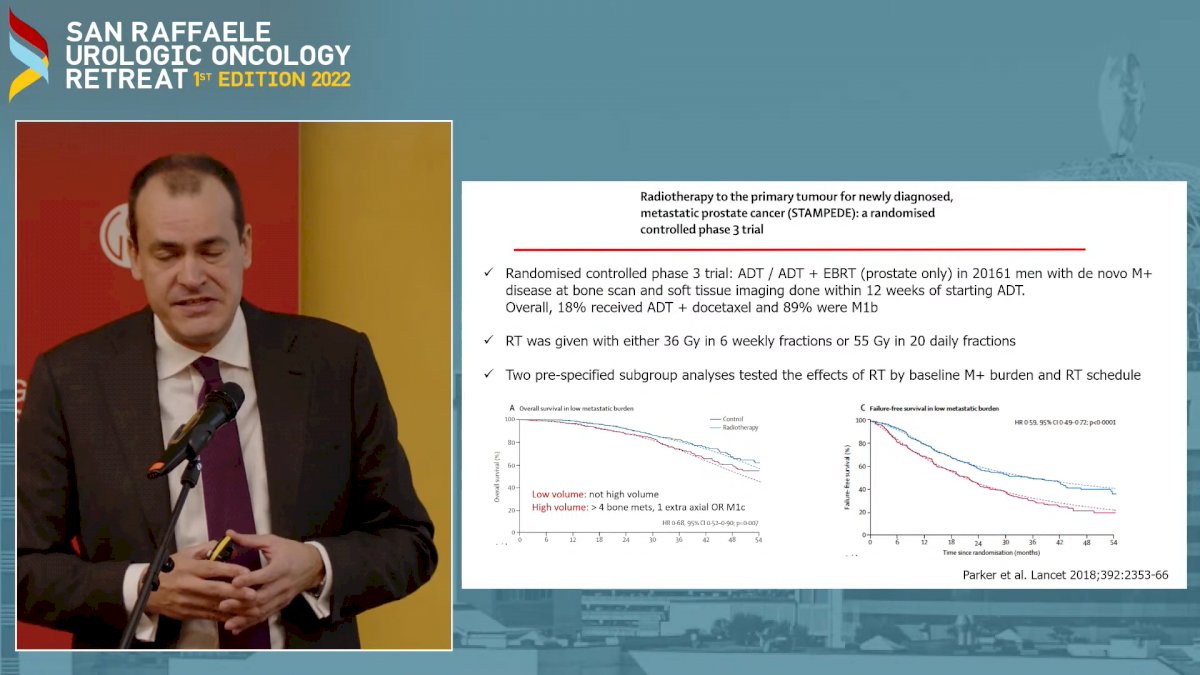
Then Prof. Briganti closed his talk by presenting the results from the STAMPEDE trial on newly diagnosed low-burden metastatic PCa about upfront RT to the primary tumor, showing that RT in this setting “significantly improved patients’ outcomes” (Fig. 2)1
After Prof. Briganti’s talk, Prof Andrea Necchi went up on the stage and started by showing a chronological chart of how in the last decades the State of the art in bladder cancer changed dramatically. Starting from the introduction of BCG therapy in 1976, till the recent approval of novel Immune-checkpoint inhibitors and novel target-therapy agents.

He remembered the Phase I study conducted by Powles, Vogelzang, et al on metastatic bladder cancer patients and the first “hints on efficacy” of anti-PDL1 therapy in this setting, defined as “ the first major step in the recent story of immunotherapy in bladder cancer.
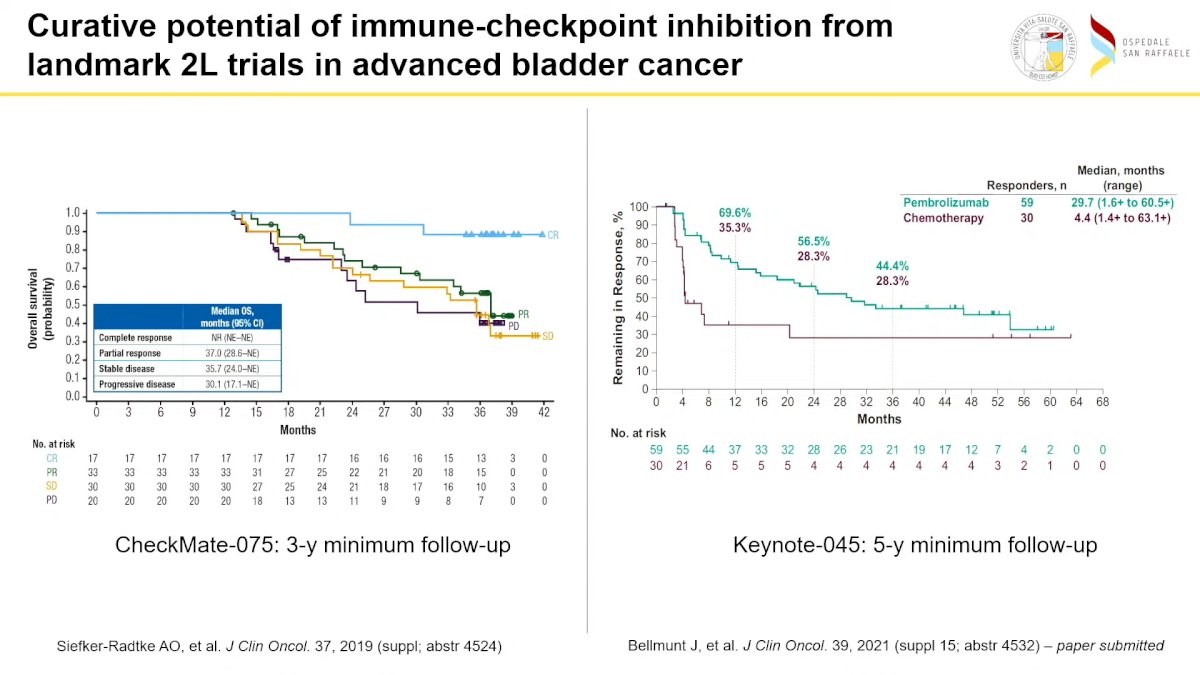
Then he continued showing the IMvigor-210 Phase II study of Atezolizumab in metastatic urothelial carcinoma (UC) conducted by Rosenberg and Dreicer, which led to the accelerated FDA approval of Atezolizumab single agent in advanced bladder cancer. Prof. Necchi continued his presentation by showing Keynote-045, “third pillar in the history of Immunotherapy development” providing evidence of the significant advantage of Pembrolizumab in 2nd line compared to SOC CT in patients with advanced bladder cancer, laying the foundation for a new SOC. Prof. Necchi then showed the long-term data (3 years for the CheckMate 075 and 5 years for Keynote-045) underlying that even in the advanced settings a proportion of patients could obtain a cure.
After showing the “explosion” of different studies since 2016, he displayed the disappointing results of 3 phase III studies comparing IO + CT vs IO alone vs CT in advanced/metastatic bladder cancer (IMvigor 130, DANUBE, and Keynote-361) which failed to demonstrate a PFS nor an OS benefit of the combination arm (IO+ CT) Prof. Necchi continued showing the results of the JAVELIN trial, conducted by Powles and Grivas in which sequential therapy, CT first and than Avelumab as maintenance in those patients not progressing after CT met his primary OS endpoint as the first line in advance/metastatic UC. These results were confirmed also by the 2 years follow-up of this study.2 He continued underlying the yet unmet need for an “ ideal biomarker to select patients which could benefit the most from IO therapy” showing the largely inconsistent data and results across different studies evaluating TMB or CPS

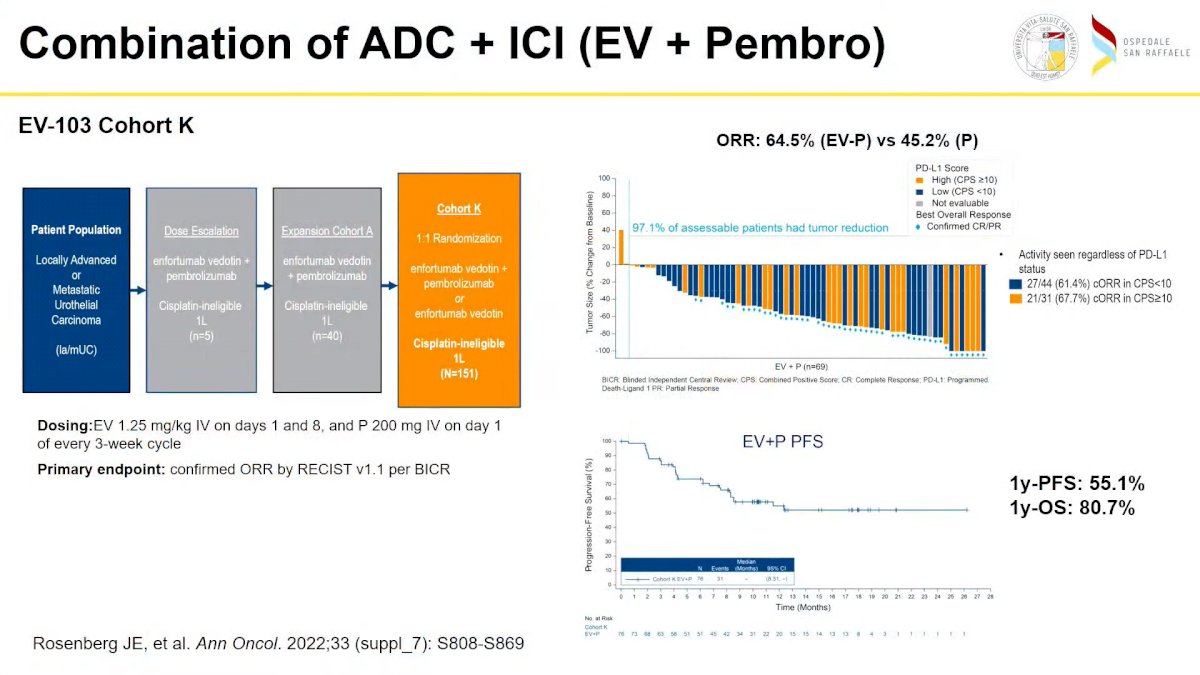
After showing the results of the BLC2001 study conducted by Loriot et al, which led to FDA approval of erdafitinib for locally advanced UC, he presented the results which led to the FDA approval post-PD-1 or PD-L1 and CT of ADC Enfortumab vedotin (EV), widening the different treatment options for UC. Then he showed the “unbelievably outstanding results” of the combination of EV + pembrolizumab as the first line in locally advanced or metastatic UC, presented recently by Rosenberg et Al with an ORR of 64.5%
The focus of the talk moved to the early stages of UC displaying the design and results of the pioneering PURE-01 study, conducted by Prof. Necchi in 2018, which established a major milestone for IO therapy in this setting. He continued showing different trials in the same setting, based on the model of PURE-01, but underlying that “we are on the right way, but still far from a standard of care”. Thereafter he presented the results of Phase III checkmate 274, where Nivolumab as an adjuvant single agent after radical cystectomy met his primary endpoint showing a benefit in DFS compared to placebo in this setting and representing “ another major pillar in this disease”. He then mentioned the “complete revolution” represented by the Keynote-057 study, where Pembrolizumab demonstrated promising antitumor activity in the setting of nonmuscle invasive bladder cancer unresponsive to BCG. Prof Necchi ended his talk by showing the significant worldwide geography discrepancy of treatment’s availability and approval due to reimbursement and financial issues, and the “future gaps” which should be filled: safety/efficacy threshold, the optimal therapy sequence, the central role of the patient in the decision-making process to address the right treatment for them.

The last speaker of this section was Dr. Umberto Capitanio, who opened his talk by showing the major changing in the state of the art from 2012 and 2022 in the different settings of Kidney cancer. In the EAU guidelines of 2012 surgical therapy (open and laparoscopic) was the only curative therapeutic approach considered for the treatment of RCC, in the same setting, 2022 active surveillance is a valid option and in well-selected patients, we can aspect very low growth-rate, with less than 20% of patients having a fast-grow rate, and that after the first year the growth rate trends to stabilize, and by that “with an optimal selection the cancer-related mortality in this patients is virtually zero”, said Dr. Capitanio.

He then continued speaking about the use of different focal therapies, worth mentioning the interesting results of a multicenter prospective trial, analyzing the 5 years outcomes of stereotaxic radiotherapy for patients with stage I and stage II kidney cancer. In this study, stereotaxic RT was compared to surgery, showing “ a very similar cancer-specific survival, with virtually no grade 3 toxic effect nor treatment-related deaths and neither significant worsening of the renal function” said Dr. Capitanio.

Speaking about Stage III, Dr. Capitanio showed that since 2012 different clinical trials with different TKI, failed to demonstrate any significant benefit as adjuvant therapy, but this year Powles et Al presented the updated result of Keynote-564 and the sustained benefit in DFS after 30 months follow-up with an HR of 0.63 of Pembrolizumab vs Placebo as post-nephrectomy adjuvant therapy in patients with a high risk of recurrence.
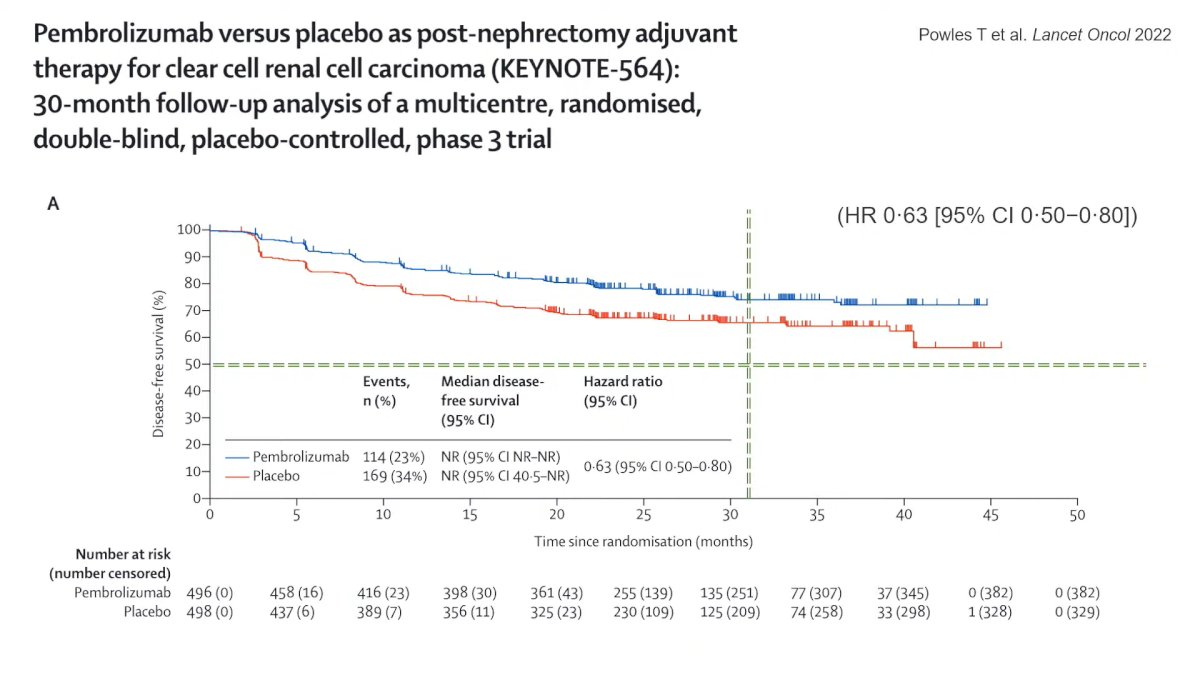
Also, for stage IV big changes occurred in the last 10 years, cytoreductive nephrectomy was recommended in this setting in 2012, but according to CARMENA trial demonstrate that Sunitinib alone was not inferior compared to Sunitinib plus nephrectomy. Nowadays Sunitinib is no more the SOC first line, where it is represented by novel combination therapies of IO/TKI or IO/IO.
Dr. Capitanio closed his talk by showing how also the focus of the research in the Urological field changed. 10 years ago urological research was mainly focused on the surgical aspect, while currently functional outcomes, frailty, and hospital quality, which were almost completely neglected 10 years ago, play key roles in modern research.
“In the last 10 years so many things have changed, and probably in the next 10 years things will, even more, change”, said Dr. Capitanio closing his presentation and the first session of this Meeting Chaired by Prof. Montorsi.
Presented by:- Alberto Briganti, MD, Ph.D. Urology San Raffaele University Hospital, Milan, Italy
- Andrea Necchi, MD, Professor, Oncology, San Raffaele University Hospital, Milan, Italy
- Umberto Capitanio, MD, Urology San Raffaele University Hospital, Milan, Italy
References:
- Parker et al., Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet (London, England). 2018 Oct 18. doi: 10.1016/S0140-6736(18)32486-3.
- Powles et al., Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (aUC): Long-term outcomes from JAVELIN Bladder 100 in subgroups defined by response to 1L chemotherapy. Journal of Clinical Oncology 40, no. 16_suppl (June 01, 2022) 4559-4559.


