In the next section, some technical aspects of radical prostatectomy were discussed, and important tips and tricks were given as well, to improve the way this surgery is performed, both open and robotically. The first topic discussed was urinary continence. For patients choosing to undergo radical prostatectomy, regret is most commonly associated with their urinary incontinence.3 Urinary continence after radical prostatectomy is influenced by the patient’s age, the previous local treatments he had received, and the nerve-sparing he had undergone in surgery. An important technical point is individualizing the apical dissection. A multivariable analysis demonstrated that only a specific prostate apical shape (Figure 1) was associated with early continence after radical prostatectomy. Additionally, preservation of the maximal possible functional length of the urethral sphincter during radical prostatectomy is critical as well, as seen in figure 2. For the surgeon, it is also important to track and record what he did in each case to make sure that is an improvement is made over time. Most importantly, patient selection is critical, and older patients with a higher risk for urinary incontinence (patients undergoing salvage radical prostatectomy, patients with a short membranous urethra, and those who have had a previous TURP), should be referred for other procedures such as focal therapy and radiotherapy, due to their increased risk of urinary incontinence.
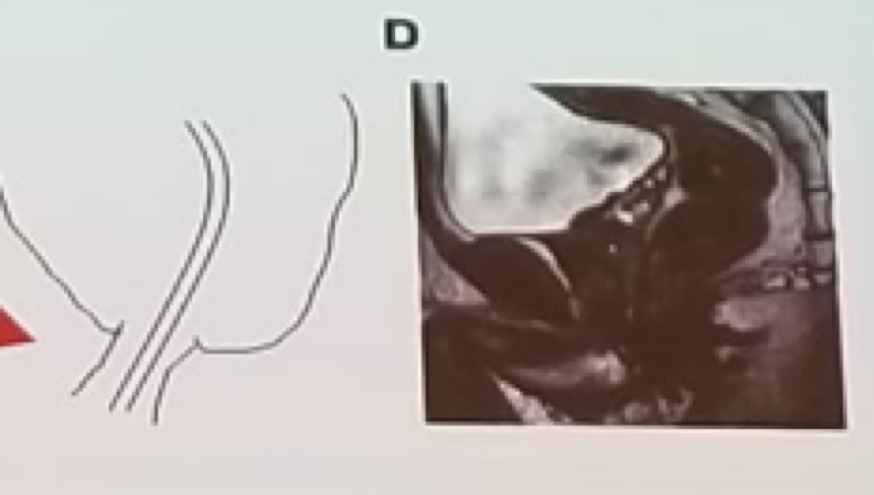
Figure 1 – Prostate apex shape associated with the early return of continence after surgery:
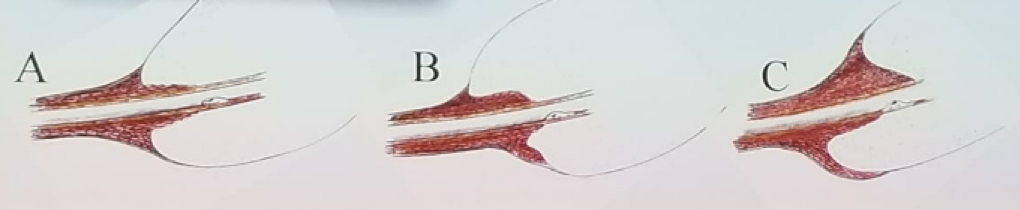
Figure 2 – The importance of maximal functional preservation of the urethral sphincter near the prostate apex:
The length of the membranous urethra and the extent of the nerve-sparing, have been shown to predict post-prostatectomy continence.4 Every millimeter of membranous urethral length that is spared, improves continence by 9%!5 Furthermore, the concept of nerve sparing during surgery was also mentioned in this context. It has been shown that nerve-sparing not only improves the erectile function but also the long-term continence rates. Therefore, the true organ-confined disease needs to be recognized, and nerve-sparing needs to be performed even in men who are not sexually active.
Another important concept is the performance of a posterior reconstruction, the so-called “Rocco stitch,” which entails the posterior reconstruction of the Denonvilliers’ musculofascial plate. Although two comparative studies have shown no benefit in this technique, many urologists still perform this stitch during radical robotic prostatectomies.
The preservation of potency was the last topic discussed. Recognizing and understanding the anatomy is the first crucial step. Additionally, it is important to avoid using energy or traction around the neurovascular bundles. To improve the nerve-sparing technique, the use of neurovascular structure-adjacent frozen section examination (Neurosafe) was recommended. This has been demonstrated to increase the success of nerve sparing and reduce the positive surgical margins rate in both open and robotic radical prostatectomies.7 Understanding the anatomy will assist in understanding the difference and the implications of an intrafascial, interfascial, or extrafascial approach. In an intrafascial approach, a full nerve-sparing technique is performed, while in interfascial we perform only a partial nerve sparing, and finally, in extrafascial technique, nerve-sparing is not performed, as depicted in figure 3. The nerve-sparing procedure should start with an anterior high release of the nerve bundles from the prostate, as it has been shown that nerves at the ventral prostatic capsule contribute to erectile function.8
Lastly, the role of penile rehabilitation after surgery was discussed, which is still quite controversial and debatable. To date, no specific recommendation can be given regarding the structure of the optimal rehabilitation regimen, although data suggest that there is no difference between on demand and daily PDE5Is treatment for erectile function recovery. Currently, there are many erectile function rehabilitation programs using PDE5Is, intracavernosal injections, and vacuum therapy. However, there is no clear-cut evidence supporting their usage.
In conclusion, patient selection and counseling regarding the postoperative functional outcomes is crucial. Accurate anatomical dissection and reconstruction are critical to achieving maximal results.
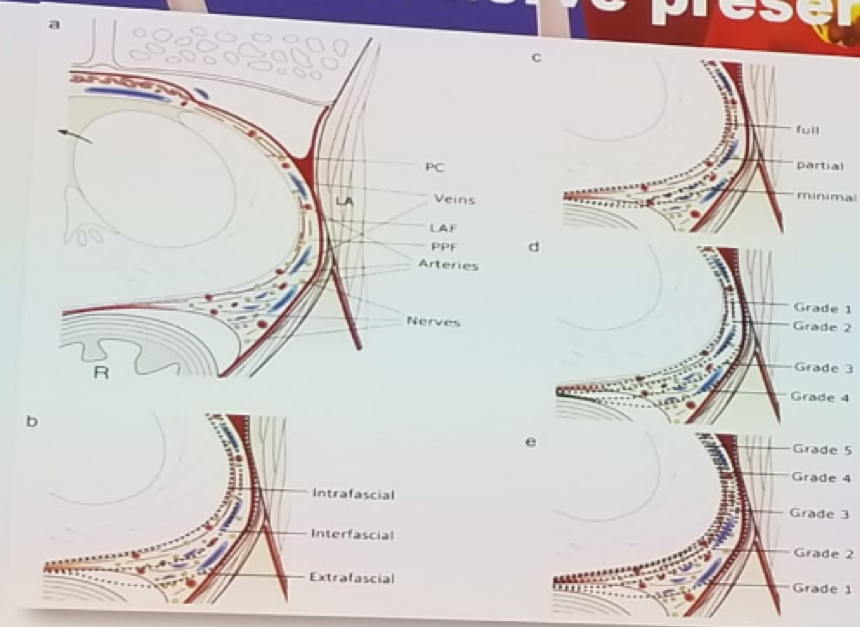
Figure 3: Extent of nerve sparing and its correlation to the different dissecting planes:
Presented by: Graefen, Hamburg, Germany H. van der Poel, Amsterdam, Netherlands
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the 2nd EAU Update on Prostate Cancer (PCa18)– September 14-15, 2018 – Milan, Italy
References:
1. Hamdy et al. NEJM 2016
2. Wallis et al. Eur Urol 70 (2016) 21-30
3. Schroeck et al. Eur Urol 54: 785, 2008
4. Mungovan et al. Eur Urol 2017, Marl 71 (3): 368-378
5. Grivas et al. Neurourol Urodyn 2018
6. Grasso AAC et al. BJUI 2016
7. Schlomm T et al. European urology 2012; 62(2): 333-40.
8. Kaiho Y et al. European urology 2009; 55(1): 148-54.
Another important concept is the performance of a posterior reconstruction, the so-called “Rocco stitch,” which entails the posterior reconstruction of the Denonvilliers’ musculofascial plate. Although two comparative studies have shown no benefit in this technique, many urologists still perform this stitch during radical robotic prostatectomies.
The preservation of potency was the last topic discussed. Recognizing and understanding the anatomy is the first crucial step. Additionally, it is important to avoid using energy or traction around the neurovascular bundles. To improve the nerve-sparing technique, the use of neurovascular structure-adjacent frozen section examination (Neurosafe) was recommended. This has been demonstrated to increase the success of nerve sparing and reduce the positive surgical margins rate in both open and robotic radical prostatectomies.7 Understanding the anatomy will assist in understanding the difference and the implications of an intrafascial, interfascial, or extrafascial approach. In an intrafascial approach, a full nerve-sparing technique is performed, while in interfascial we perform only a partial nerve sparing, and finally, in extrafascial technique, nerve-sparing is not performed, as depicted in figure 3. The nerve-sparing procedure should start with an anterior high release of the nerve bundles from the prostate, as it has been shown that nerves at the ventral prostatic capsule contribute to erectile function.8
Lastly, the role of penile rehabilitation after surgery was discussed, which is still quite controversial and debatable. To date, no specific recommendation can be given regarding the structure of the optimal rehabilitation regimen, although data suggest that there is no difference between on demand and daily PDE5Is treatment for erectile function recovery. Currently, there are many erectile function rehabilitation programs using PDE5Is, intracavernosal injections, and vacuum therapy. However, there is no clear-cut evidence supporting their usage.
In conclusion, patient selection and counseling regarding the postoperative functional outcomes is crucial. Accurate anatomical dissection and reconstruction are critical to achieving maximal results.

Figure 3: Extent of nerve sparing and its correlation to the different dissecting planes:
Presented by: Graefen, Hamburg, Germany H. van der Poel, Amsterdam, Netherlands
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the 2nd EAU Update on Prostate Cancer (PCa18)– September 14-15, 2018 – Milan, Italy
References:
1. Hamdy et al. NEJM 2016
2. Wallis et al. Eur Urol 70 (2016) 21-30
3. Schroeck et al. Eur Urol 54: 785, 2008
4. Mungovan et al. Eur Urol 2017, Marl 71 (3): 368-378
5. Grivas et al. Neurourol Urodyn 2018
6. Grasso AAC et al. BJUI 2016
7. Schlomm T et al. European urology 2012; 62(2): 333-40.
8. Kaiho Y et al. European urology 2009; 55(1): 148-54.


