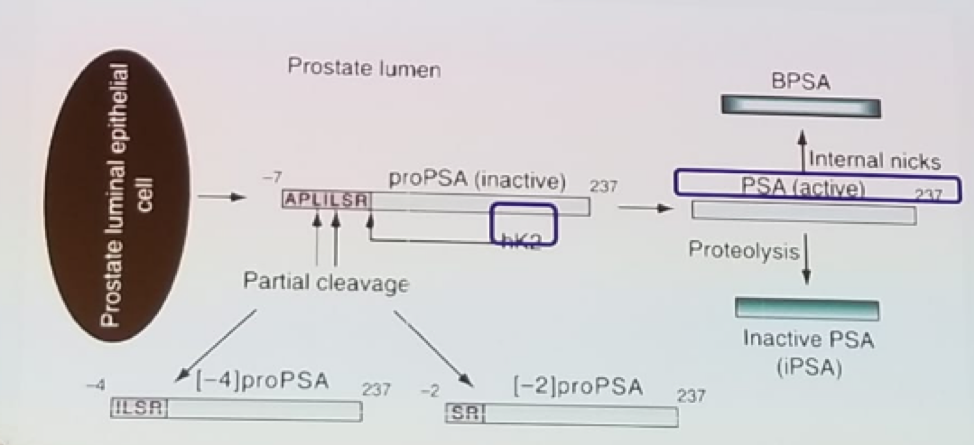
Figure 1 – PSA production:
PSA has been shown to have a direct correlation to prostate cancer risk. As the PSA rises, the risk of prostate cancer increases accordingly1. Traditionally a cut off of 3 ng/ml was considered the classic cutoff from normal to a high risk of prostate cancer diagnosis, as can be seen in figure 2. A rise in age is correlated with an increased risk of benign prostatic hyperplasia (BPH), and prostate cancer. At age 20-30 the risk of prostate cancer is 2%, and it rises to 64% at age 60-70.
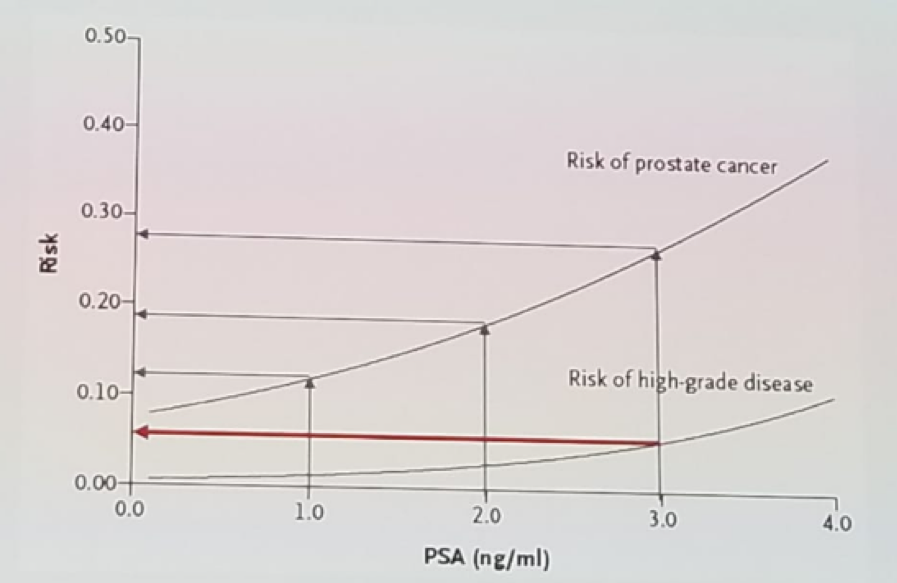
Figure 2 – Correlation of PSA levels to prostate cancer risk
The speakers then discussed the role of the ratio of free/total PSA. This ratio is used in stratifying the risk of prostate cancer in men with a PSA between 4 ng/ml and ten ng/ml, and a negative digital rectal examination. It has been shown that the risk of prostate cancer is 56% if the free to total ratio is <0.1, and the risk is 8% if the ratio is >0.252. The free/total ration has no use during follow-up of known prostate cancer when the total PSA is below three ng/ml, and when it is above 20 ng/ml.
There is a known variation with the accredited (WHO-calibrated) methods of PSA testing of +/-5%. However, there is also a known intra-individual variation of +/-15%, which physicians and patients need to remember. There are also several reasons for a falsely elevated PSA level. These include urinary tract infections (the PSA can take up to a year to normalize!), urinary retention, and a prostate biopsy. Additional sources that can cause an elevated PSA include chronic renal failure, hypogonadism, and mishandling of blood samples analyzed for PSA.
PSA doubling time is an important parameter that we need to comprehend. This is based on PSA values analyzed over time. A minimum of two points are required, but more than 2 points are considerably more reliable. The equation for calculation of doubling time from two PSA values is the following: PSA doubling time = [ln(2)*/IT]/[ln(PSA final) – ln(PSA initial)]. The PSA must be measured at least four weeks apart for the doubling time calculation. The minimal increase between the two values must be 0.4 ng/ml. The consensus today is that at least three values are required of the proper calculation of PSA doubling time. There is also an online tool that can assist in the calculation of the doubling time.
Next, the large European Randomized study of Screening for Prostate Cancer (ERSPC) screening trial was discussed. This was a randomized controlled multicenter trial including eight centers, with men aged 55-69. In the intervention group, screening with PSA was performed every four years. Overall 182,160 men were screened, with 72891 men in the intervention group, and 89352 men in the control group. This trial demonstrated a clear advantage in prostate cancer mortality for patients in the screening intervention group. After follow-up for 13 years, screening was shown to have a beneficial effect on prostate cancer mortality, with a decrease of 21%, and a rate ratio of 0.79 (95% C.I 0.69-0.91). However, to save one life, the number needed to screen was 781, while the number needed to diagnose was 27. These numbers decrease as follow-up time was longer.
This demonstrated how overdiagnosis and overtreatment are real issues in prostate cancer. The Gothenburg branch of ERSPC study had a follow-up period of 18 years3, for 20000 men. This demonstrated a number needed to screen of 231, and a number needed to diagnose of 10, with a reduction of 35% in the risk of death. Amazingly, these numbers are better than those accepted for other types of cancers, with an accepted screening program, such as breast (number needed to screen 100-2000, with number needed to diagnose of 10), cervical cancer (number needed to screen 1140, and unknown number needed to diagnose), and colorectal cancer (number needed to screen of 600-1200, with an unknown number needed to diagnose).
A previously mentioned, over-diagnosis is a serious problem, as it drives up healthcare costs, results in “labeling” healthy people as patients with cancer, and ultimately leads to overtreatment, which in turn reduces the quality of life of the patient. This problem needs to be managed, and here they discussed a few steps that can assist us with this. The opinions shared include:
First, we should consider stopping PSA screening of men with a life expectancy of fewer than 10-15 years.
Second, we need to risk stratify screening based on age, comorbidities, and PSA levels.
Third, we need to sharpen our diagnostics through sequential screening. For instance, PSA followed by multiparametric MRI, and then perform a targeted biopsy. Lastly, we need to organize information/screening/PSA-testing programs.
The next topic discussed was PSA relapse after definitive therapy for the local disease. PSA level is a sensitive marker of occult prostate-cancer relapse and provides early notification of recurrence, but a PSA relapse does not equal a clinical relapse or death from prostate cancer. The definition of relapse after prostatectomy is a consecutively rising prostate-specific antigen (PSA) values of >0.2 ng/mL. PSA measurements after radiation are even less predictable. After external beam radiotherapy (EBRT) the Phoenix definition is used to define PSA relapse. In the post-radiation therapy setting, the most widely accepted definition is a PSA that is seen to be rising from the lowest level (nadir) by at least 2.0 ng/mL. Factors that have been shown to predict systemic relapse include pathological T3b, positive lymph nodes on pathology, Gleason score >=7, relapse that occurs in less than two years from definitive treatment, and PSA velocity at relapse of more than 0.75 ng/ml/a year. Several studies have shown that PSA doubling time thresholds of between less than six months and less than ten months are significant predictors suggesting a systemic, rather than a local recurrence.
Figure 3 demonstrates the correlation of PSA to time to survival, showing a hazard ratio of 19.6 if the PSA doubling time is less than three months4. In post-radical prostatectomy patients, a short PSA doubling time is the key driver, with shorter doubling times correlated to a greater impact. In post EBRT, early relapse (<1 year) has the highest risk impact.
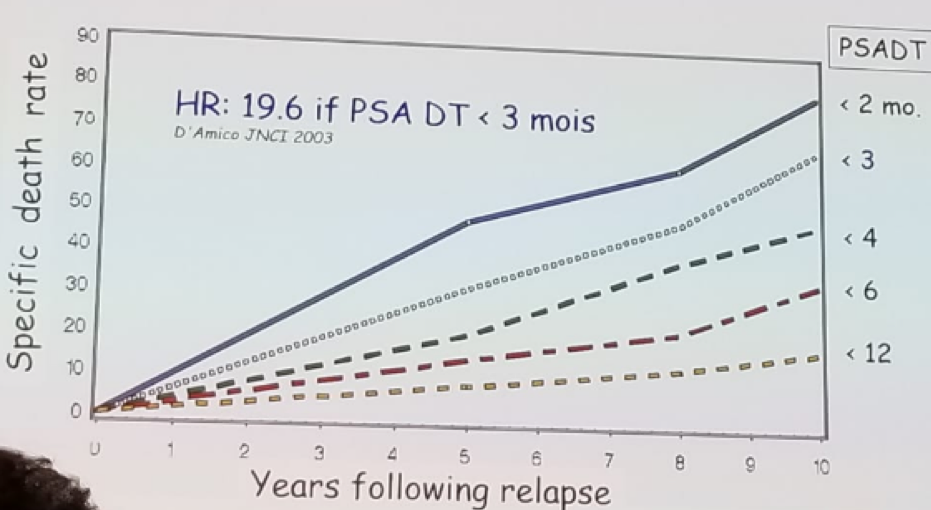
Figure 3 – Correlation between PSA doubling time (PSADT) and survival:
The speakers moved on to discuss the PSA response in M1 patients and those treated with androgen deprivation therapy (ADT). It has been shown that 7 months after beginning ADT therapy, if the PSA is higher than 4 ng/ml, the median survival is 13 months, if it is lower than 4 ng/ml the median survival rises to 44 months, and if it is lower than 0.2 ng/ml, the median survival elevates to a staggering 75 month5.
When assessing the role of PSA progression in castrate-resistant prostate cancer (CRPC), PSA reflects the androgen axis activity. It is linked to the disease when the axis is predominant (hormone-sensitive disease) but is quite different in CRPC status. In CRPC, PSA decline has been linked to increased survival, and when the decline is less than 30% after one month of treatment, it was shown to be associated with poor survival6. In non-metastatic CRPC PSA doubling time is correlated with time to metastases. Some have suggested that a PSA rise in CRPC patients should trigger a treatment change. However, treatment for CRPC should be changed only if signs for unequivocal progression exist. This is defined by at least two of the following criteria: PSA progression, bone scan progression, CT progression, and quality of life symptom progression.
In summary, PSA has a major role in all stages of prostate cancer, from diagnosis to end-stage disease. In the early diagnosis of the disease, it is limited by a lack of specificity. In local treatment follow-up, it is important to remember that PSA rise does not equal immediate treatment, and that not every rise, is a clinically significant rise. Lastly, in systemic therapy, PSA progression is but one of many other available signs of progression, and alone it is not enough to define unequivocal progression.
Presented by: Johan Stranne, Göteborg, Sweden and Nicolas Mottet, Saint-Étienne, France
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the 2nd EAU Update on Prostate Cancer (PCa18)– September 14-15, 2018 – Milan, Italy
References:
1. Thompson et al. NEJM 2004
2. Catalona, W.J. et al. JAMA, 1998
3. Hugosson J et al. Scan J urol 2018
4. D’Amico et al. JNCI 2003
5. Hussain et al. J Clin Oncol 2006
6. Resciano et al. ASCO 2018


