(UroToday.com) The 2022 IKCS North American annual meeting featured a session on multidisciplinary team approaches to rare subtypes, including a presentation by Dr. Allison May discussing spatial molecular imaging to profile the epithelial to mesenchymal transition and immune crosstalk in sarcomatoid renal cell carcinoma (RCC). Sarcomatoid RCC is thought to arise from an epithelial to mesenchymal transition of the parental tumor, most commonly clear cell RCC. These tumors are present in 5-10% of RCC and ~20% of metastatic RCC, with an associated poor prognosis of 3-13 months. These tumors are known to be highly immunogenic, however, whether the epithelial to mesenchymal transition state impacts the immune milieu and responsiveness to immunotherapy, is unknown. This study explored the capacity of spatial molecular imaging to dissect the tumor immune microenvironment and epithelial to mesenchymal transition in sarcomatoid RCC.
Nanostring’s spatial molecular imaging platform, CosMx, was used to spatially capture single cell level transcriptomic data in two sarcomatoid RCC specimens, one from a responder to immunotherapy and one from a non-responder. Fields of view within sarcomatoid, clear cell and transition areas were selected using H&E and further segmented with morphology markers SYTO11, PanCK, CD3, and CD45. Dr. May and colleagues compared regions within each tumor and the two specimens. An overview of the workflow is as follows:
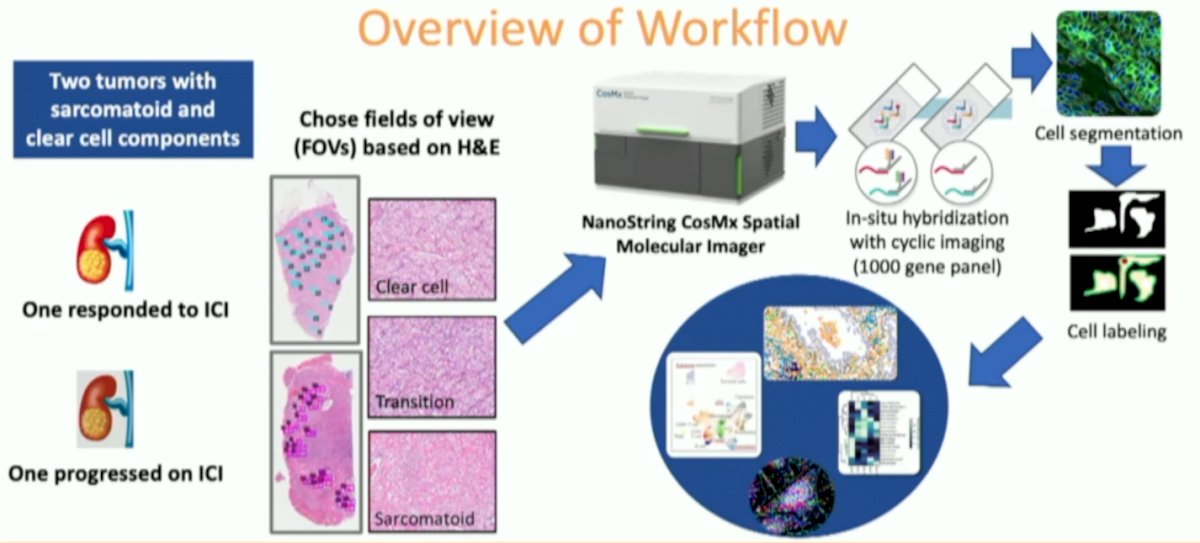
Forty fields of view and over 100,000 single cells were captured. Epithelial staining was high in clear cell regions and decreased to near absent in the sarcomatoid regions. Distinct tumor cell clusters and differing immune cell types existed between clear cell and sarcomatoid areas. Clustering revealed shared tumor cell populations between the responder and non-responder as well as unique populations in each:
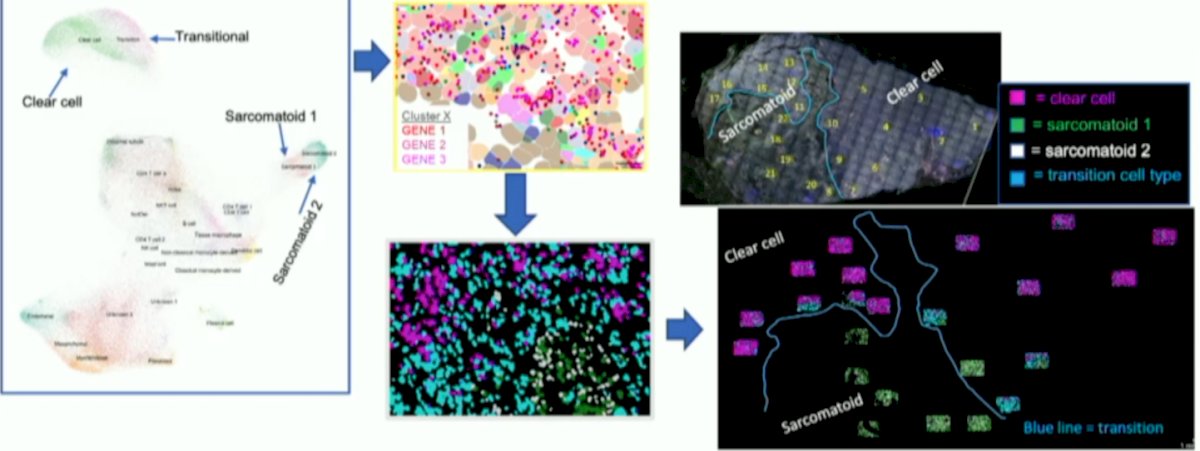
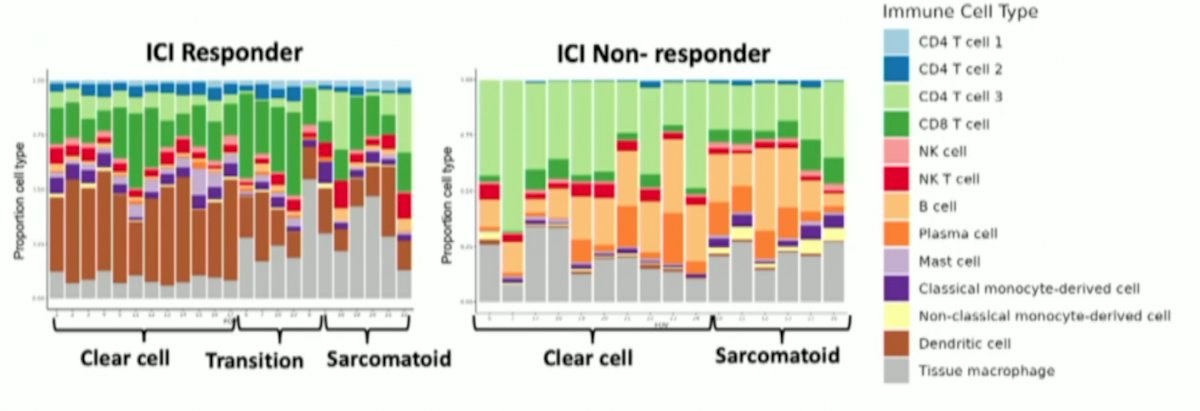
In the sarcomatoid regions, immune infiltrate was dispersed in the non-responder, but clustered in perivascular regions in the responder. CD4+ naïve T cells and myeloid dendritic cells were higher in the responder while CD4+ memory cells, CD8+ naïve T cells, and plasmacytoid dendritic cells were more abundant in the non-responder:
Dr. May concluded her presentation by discussing spatial molecular imaging to profile the epithelial to mesenchymal transition and immune crosstalk in sarcomatoid RCC with the following take home messages:
- The combination of single cell and spatial resolution is a powerful tool for studying RCC, leading to an improved understanding of tumor biology and new therapeutics
- A subset of morphologically-classified clear cell RCCs have gene expression indicating progression, which is a future biomarker
- Sarcomatoid RCC tumors have spatial intra-tumoral heterogeneity of tumor cell and immune populations
Presented by: Allison May, MD, University of Michigan, Ann Arbor, MI
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 International Kidney Cancer Symposium (IKCS) North America, November 4-5, Austin, Texas, USA


