(UroToday.com) The second poster session at the 2022 International Kidney Cancer Symposium (IKCS): North America meeting featured a number of presentations, including one from Dr. McKay discussing randomized data from the RADICAL trial of radium-223 and cabozantinib among patients with advanced renal cell carcinoma (RCC) with bone metastases.
Bone metastases are relatively common in patients with advanced RCC, affecting approximately 30% of patients. Compared to others with advanced RCC, those with bone metastases have a worse prognosis and are at risk of symptomatic skeletal events (SSEs). The vascular endothelial growth factor (VEGF) receptor and MET kinase inhibitor cabozantinib has demonstrated improved survival in patients with RCC and has enhanced activity in bone. Radium-223, an alpha-emitting radioisotope with natural bone-seeking proclivity, has prolonged survival in men with advanced prostate cancer. In a prior pilot study of Ra-223 with VEGF inhibition in patients with RCC and bone metastases, this regime demonstrated safety and declines in markers of bone formation and resorption. Given that decreasing rates of SSEs and improving outcomes are unmet needs in patients with RCC and bone metastases, we designed a randomized phase 2 study through the National Clinical Trials Network (NCTN) investigating cabozantinib with or without Radium-223 in pts with RCC with bone metastases.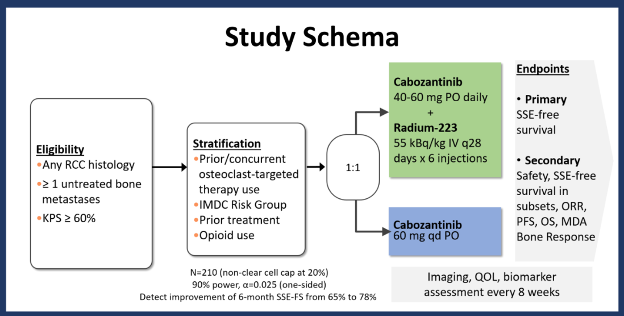
This open-label, multicenter trial is enrolling eligible patients with metastatic RCC of any histology who have at least one metastatic bone lesions untreated with prior radiation therapy and any number of lines of prior therapy. Patients with non-clear cell RCC are eligible, though their inclusion is capped at 20% of accrual. Patients must have a Karnofsky performance status of ≥60% and be on osteoclast-targeted therapy unless otherwise contraindicated.
Methodologically, patients are randomized 1:1 to cabozantinib with (Arm A) or without (Arm B) Ra-223. The starting dose of cabozantinib for Arm A is 40 mg to be escalated to 60 mg daily after cycle 1 (1 cycle=28 days) if there is no persistent grade 2/≥3 toxicity. Radium-223 is administered at a fixed dose of 55 kB/kg IV every 28 days x 6 doses.
The primary endpoint is SSE-free survival. Secondary endpoints include safety, progression-free survival, overall survival, quality of life measures, and correlative analyses including liquid biopsy studies and tumor tissue analysis.
Presented by: Rana R. McKay MD, University of California San Diego, La Jolla, CA


