(UroToday.com) The International Kidney Cancer Symposium 2021 annual hybrid meeting included a Keynote Address by 2018 Nobel Prize laureate Dr. James Allison who discussed immune checkpoint blockade in cancer therapy. Dr. Allison started his talk by noting that in 1996, based on data regarding signals that regulate T cell responses, Dr. Allison hypothesized that tumor cells, which do not express B7 molecules, have a head start against the immune system given that T cells can not initially recognize tumor cells. However, as tumors grow and a few tumor cells died, antigen presenting cells are able to phagocytose the dead tumor cells and present mutated tumor antigens via MHC to T cells. Subsequently, T cells would interact with antigen presenting cells, in the context of T cell receptor plus MHC plus antigen and CD28 plus B7. This would subsequently lead to T cell activation consisting of proliferation and cytokine production, and lead to activated T cells focused on tumor eradication, as summarized in the following figure:

However, CTLA-4 halts the T cell response in order to prevent T cells from continuous proliferation and cytokine production. Dr. Allison notes that he thought that if CTLA-4 halted T cell responses too soon before all tumor cells were eradicated, the tumor would win and the cancer would persist. But, an antibody to block CTLA-4 would enable T cell responses to persist long enough to eradicate all tumor cells as highlighted in the following figure:

Based on this information, Dr. Allison’s lab designed experiments in tumor-bearing mice to test whether anti-CTLA-4 antibodies would lead to tumor rejection. They found that in the presence of a CTLA-4-blocking antibody, after a short delay while the immune system ramped up, tumors would melt away:1

Based on this data, Medarex and Bristo-Myers Squibb produced the first fully human antibody to CTLA-4, ipilimumab. This CTLA-4 antibody showed objective responses in many tumor types, including melanoma, prostate, kidney, bladder, ovarian, and lung cancer, however with adverse events (colitis, hepatitis, hypophysitis, etc) that could be serious but generally manageable. Very rarely, patients may develop diabetes or myocarditis. In early studies of anti-CTLA-4 dating back to 2004, Dr. Allison notes that some patients with metastatic melanoma (which at the time had a very poor median survival of ~8 months) were complete responders to treatment. One example was a young patient who experienced complete resolution of two subcutaneous nodules, 31 lung metastases, and a 0.5 cm brain metastasis:
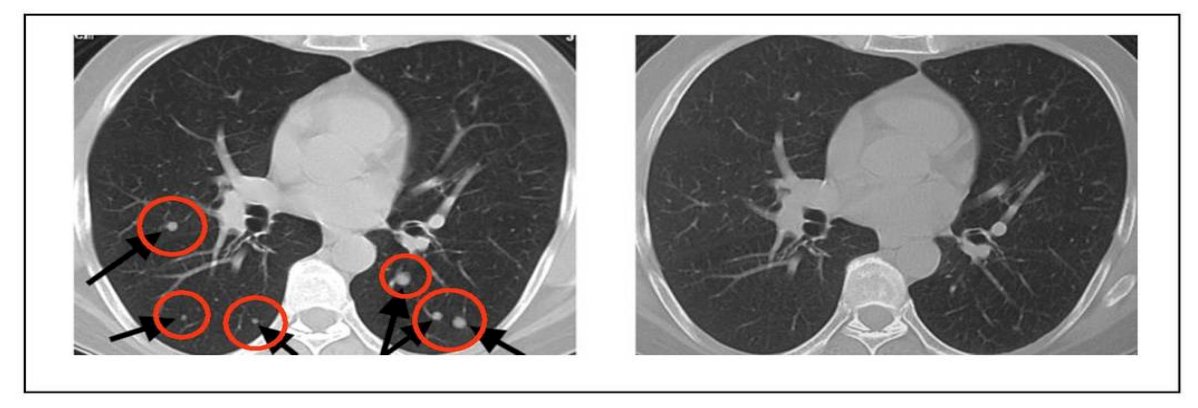
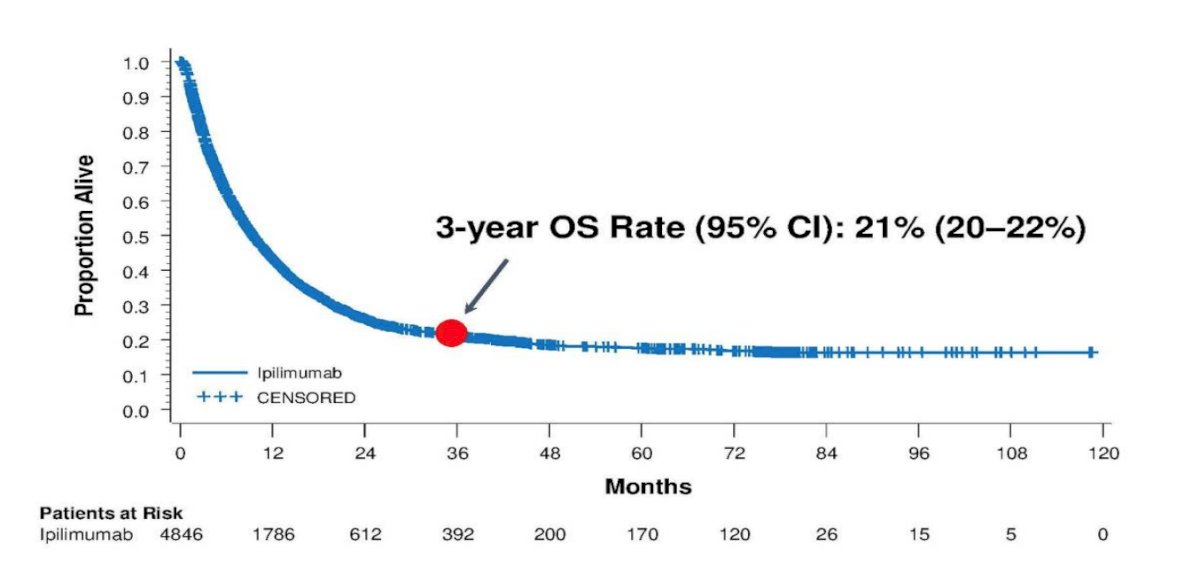
By 2010, data from the first phase 3 trial of unresectable stage III/IV metastatic melanoma demonstrated an overall survival benefit, with ~45% of patients who received anti-CTLA-4 reported to be alive at 1 year and ~23% of patients who received anti-CTLA-4 reported to be alive at 2 years.2 Subsequent data reported from a cohort of 4,846 patients treated with ipilimumab on multiple clinical trials demonstrated a 3-year OS rate of 21% (95% CI 20-22%) for patients with metastatic melanoma:3
Switching gears to anti-PD-1, Dr. Allison notes that the phase I trial of nivolumab included 296 patients with metastatic cancer with doses of 1, 3, 10 mg/kg and the maximum tolerated dose was not reached.4 Adverse events were comparable to ipilimumab, but 4% of patients had pneumonitis. With regards to clinical activity by disease site, this is summarized as follows:
- Melanoma (n = 94): 28% complete response/partial response, 6% stable disease
- NSCLC (n = 76): 18% complete response/partial response, 7% stable disease
- RCC (n = 33): 27% complete response/partial response, 27% stable disease
- Colorectal cancer (n = 19) and CRPC (n = 13): no responses
Dr. Allison notes that from here he feels that we move towards combination therapies. In a recent report of 5-year outcomes of a clinical trial assessing nivolumab + ipilimumab or nivolumab alone among patients with advanced melanoma,5 the median overall survival was more than 60.0 months (median not reached) in the nivolumab + ipilimumab group and 36.9 months in the nivolumab group, as compared with 19.9 months in the ipilimumab group (HR nivolumab + ipilimumab versus ipilimumab, 0.52; HR nivolumab versus ipilimumab, 0.63). Overall survival at 5 years was 52% in the nivolumab + ipilimumab group and 44% in the nivolumab group, as compared with 26% in the ipilimumab group:
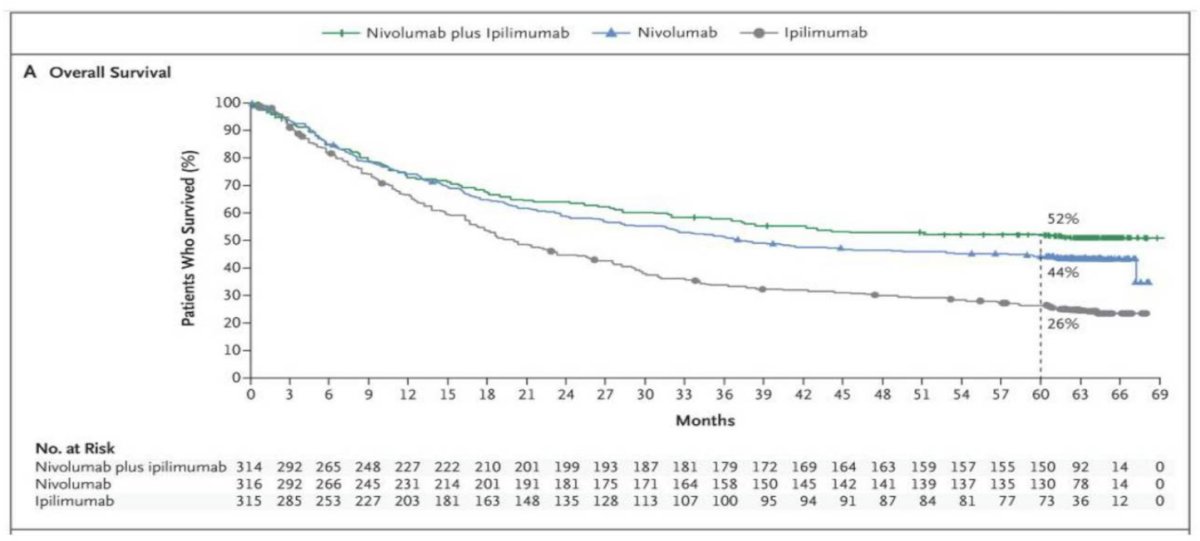
Dr. Allison notes that there are several key differences between anti-CTLA-4 and anti-PD-1 antibodies. Features of anti-CTLA-4 are as follows:
- It is hard-wired
- Targets the CD28 pathway
- Works during priming
- Expands clonal diversity
- Responses are often slow
- Primarily affects CD4 T cells
- Can move T cells into “cold” tumors
- Adverse events are relatively frequent
- Disease recurrence after response is rare
Features of anti-PD-1 are as follows:
- Induce resistance
- Target the T-cell receptor pathway
- Works on differentiated T-cells
- Does not expand clonal diversity
- Responses are usually rapid
- Only affects CD8 T cells
- Does not move T cells into tumors
- Adverse events are less frequent
- Disease recurrence after response is significant
Identifying checkpoint blockade responsive T cell populations may be possible by using CyTOF analysis of murine tumor infiltrating lymphocytes (+/- checkpoint blockade), followed by unsupervised population identification, followed by identifying associations with treatment and outcome:

Using this approach, work from Dr. Allison’s group published in 20176 used mass cytometry to comprehensively profile the effects of checkpoint blockade on tumor immune infiltrates in human melanoma and murine tumor models. They found that anti-PD-1 predominantly induces the expansion of specific tumor-infiltrating exhausted-like CD8 T cell subsets. In contrast, anti-CTLA-4 induces the expansion of an ICOS+ Th1-like CD4 effector population in addition to engaging specific subsets of exhausted-like CD8 T cells:
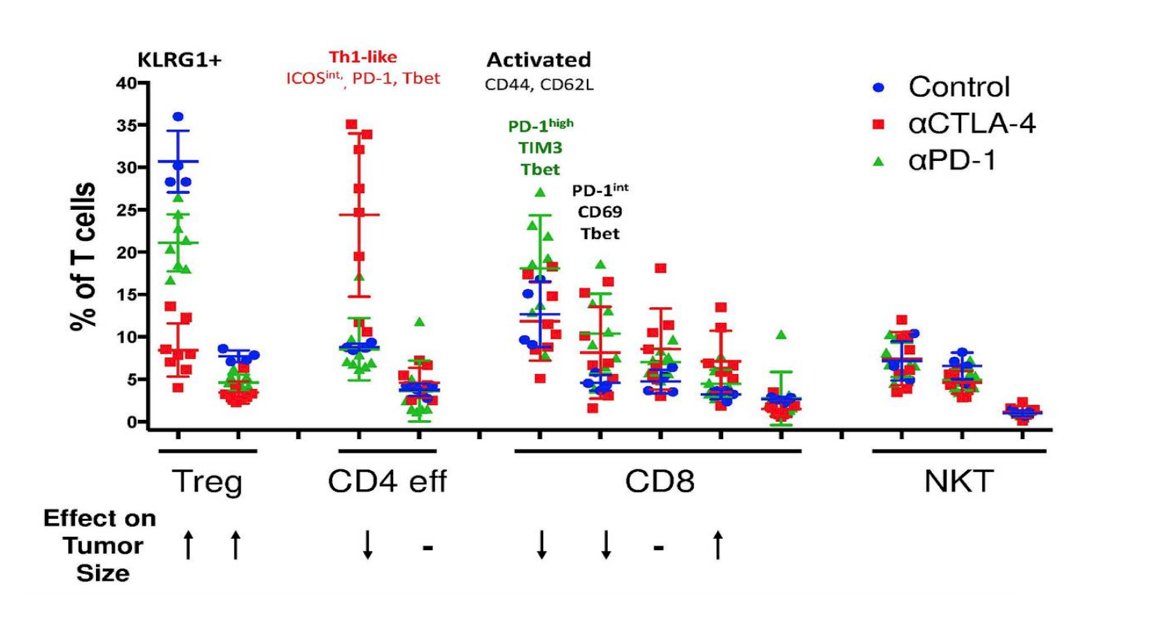
In a follow-up study from Dr. Allison’s lab in 2019,7 they further characterized the cellular mechanisms of monotherapy and combination anti–CTLA-4 plus anti–PD-1 therapy. Using high-dimensional single-cell profiling, they determined that combination therapy elicits cellular responses that are partially distinct from those induced by either monotherapy. Specifically, combination therapy mediates a switch from the expansion of phenotypically exhausted CD8 T cells to the expansion of activated effector CD8 T cells.
Based on additional unpublished data, Dr. Allison notes that there are cellular targets of checkpoint blockade, summarized as follows:
- Monotherapy
- CTLA 4:
- CD4 ICOS+ Tbet+ Th1-like effector
- CD8 Tbet+ EOMES+ KLRG-1+ effector
- PD-1:
- CD8 Tbet+ EOMES+ KLRG-1+ effector
- CD8 Tbet+ PD-1++ Lag2++ Tim3++ “exhausted”
- CTLA 4:
- Combination Therapy
- CD4 ICOS+ Tbet+ Th1-like effector
- CD8 Tbet+ EOMES+ KLRG-1+ effector
Speaking specifically with regards to immune checkpoint therapy in clear cell RCC, Dr. Allison highlights that there are both approved first-line and second-line therapies:
- First-line:
- CheckMate 2148 Nivolumab + ipilimumab: Response rate – 42%; Grade 3+ toxicities – 46%
- KEYNOTE-4269 Pembrolizumab + axitinib: Response rate – 59%; Grade 3+ toxicities – 63%
- Javelin RENAL 10110 Avelumab + axitinib: Response rate – 51%; Grade 3+ toxicities – 71%
- CheckMate 9ER11 Nivolumab + cabozantinib: Response rate – 56%; Grade 3+ toxicities – 61%
- CLEAR12 Pembrolizumab + lenvatinib: Response rate – 71%; Grade 3+ toxicities – 82.4%
- Second-line:
- CheckMate 02513 Nivolumab: Response rate – 25%, Grade 3+ toxicities – 19%
It is important that laboratories and cancer immunotherapy clinical trial groups interact, discuss and collaborate in order to answer several key questions:
- Why do some patients respond and others do not?
- Can we identify biomarkers to predict responses or toxicity?
- Are there biomarkers that would help the choice of monotherapy or combination therapy?
- How are we to increase response rates?
- Can we develop rational bases for choosing combinations?
Dr. Allison notes that in the CheckMate 025 trial assessing nivolumab versus everolimus in the second line setting, overall survival benefit was noted regardless of PD-L1 expression:13

An ongoing trial is assessing the combination of immune checkpoint blockade with surgery in metastatic clear cell RCC (n = 105), randomizing patients to Arm A (nivolumab) vs Arm B (nivolumab + bevacizumab) vs Arm C (nivolumab + ipilimumab), with the schema as follows:

Importantly, this trial of surgery + immune checkpoint therapy in the metastatic setting was associated with an OS benefit (unpublished data, details not provided). Finally, Dr. Allison highlighted work that was just published in Nature Communications of a pilot study of anti-CTLA-4 (tremelimumab) with (n = 15) or without (n = 14) cryoablation in patients with metastatic renal cell carcinoma (NCT02626130), of which 18 patients had clear cell and 11 patients had non-clear cell histologies. The trial design is as follows:

One patient in the tremelimumab arm experienced an objective response and the median progression-free survival for all patients was 3.3 months (95% CI: 2.0 to 5.3 months). Exploratory immune monitoring analysis of baseline and post-treatment tumor tissue samples showed that treatment increases immune cell infiltration and tertiary lymphoid structures in clear cell but not in non-clear cell histologies.
Dr. Allison concluded his Keynote Address discussing Immune Checkpoint Blockade in Cancer Therapy with the following figure highlighting that historically we have tried to generate a marginal survival benefit with an experimental therapy versus a standard of care or control therapy. However, with immunotherapy (ie. monotherapy anti-CTLA-4) we have improved survival more than in the past, but with the goal of additional long-term, durable survival benefits for the majority, if not all patients, with novel and well-thought-out combination therapies:

Presented by: James P. Allison, PhD, Regental Professor and Chair of Immunology, Executive Directors of the Immunotherapy Platform, Co-Director of the Parker Institute for Cancer Immunotherapy, Olga Keith Weiss Distinguished University Chair for Cancer Research, MD Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the International Kidney Cancer Symposium (IKCS) 2021 Annual Congress, November 5 and 6, 2021.
References:
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271(5256):1734–1736.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363(8):711–723
- Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33(17):1889–1894.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443-2454.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535-1546.
- Wei SC, Levine JH, Cogdill AP, et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell. 2017;170(6):1120-1133.
- Wei SC, Anang NAAS, Sharma R, et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partial distinct from monotherapies. PNAS 2019;116(45):22699-22709.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carinoma. N Engl J Med 2018;378(14):1277-1290.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1116-1127.
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1103-1115.
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021 Mar 4;384(9):829-841.
- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021 Apr 8;384(14):1289-1300.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373(19):1803-1813.
- Campbell MT, Matin SF, Tam AL, et al. Pilot study of tremelimumab with and without cryoablation in patients with metastatic renal cell carcinoma. Nat Commun. 2021 Nov 4.


