(UroToday.com) The International Kidney Cancer Symposium 2021 annual hybrid meeting included a non-clear cell renal cell carcinoma (RCC) session and a presentation by Dr. Kun-Liang Guan discussing the role of NF2 in tumorigenesis and specifically the Hippo pathway and cancer. Dr. Guan started by highlighting that the Hippo pathway plays a key role in organ size control by regulating cell proliferation and apoptosis in Drosophila, the species in which this pathway was first discovered. In work from 2007, Zhao and colleagues1 showed that in mammalian cells, the transcription coactivator YAP (Yes-associated protein), is inhibited by cell density via the Hippo pathway. Phosphorylation by the Lats tumor suppressor kinase leads to cytoplasmic translocation and inactivation of the YAP oncoprotein. Furthermore, attenuation of this phosphorylation of YAP potentiates their growth-promoting function in vivo, with YAP overexpression regulating gene expression in a manner opposite to cell density, and thus overcoming cell contact inhibition. This cellular pathway is highlighted as follows:
YAP also regulates cell fate decisions early in development. Nishioka et al.2 showed that transcriptionally active Tead4 can induce Cdx2 and other trophoblast genes in parallel in embryonic stem cells. In embryos, the Tead4 coactivator protein Yap localizes to nuclei of outside cells, and modulation of Tead4 or Yap activity leads to changes in Cdx2 expression. Subsequently, inside the cells, Yap is phosphorylated, which involves the Hippo signaling pathway component Lats. Thus, active Tead4 promotes trophectoderm development in outside cells, whereas Tead4 activity is suppressed in inside cells by cell contact- and Lats-mediated inhibition of nuclear Yap localization.
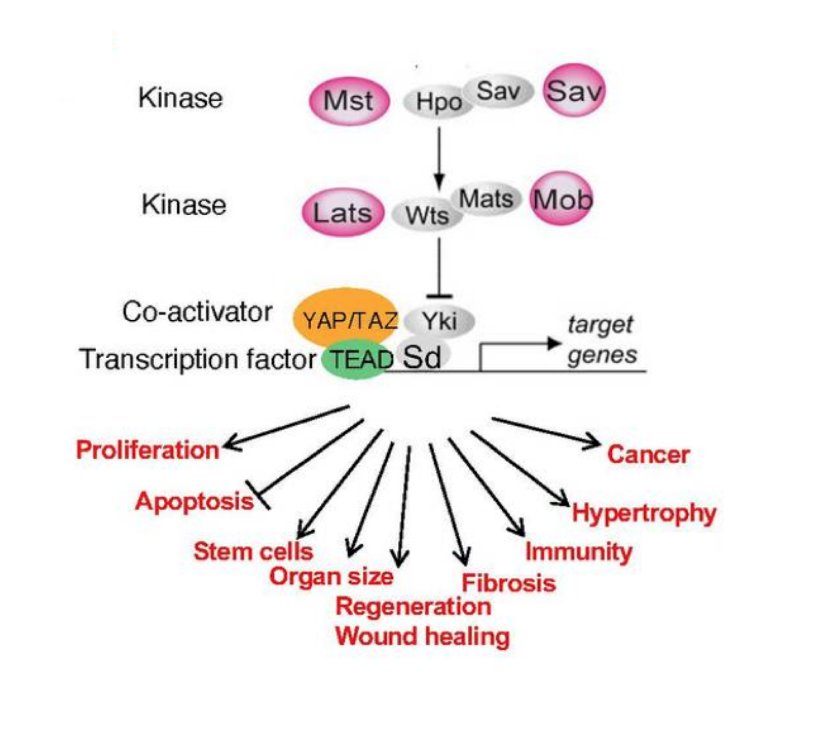
Dr. Guan notes that the Hippo pathway is among the newest signaling pathways commonly altered in human cancer. Using mutations, copy-number changes, mRNA expression, gene fusions, and DNA methylation in 9,125 tumors profiled by The Cancer Genome Atlas (TCGA), Sanchez-Vega et al.3 analyzed the mechanisms and patterns of somatic alterations in ten canonical pathways: cell cycle, Hippo, Myc, Notch, Nrf2, PI-3-Kinase/Akt, RTK-RAS, TGFβ signaling, p53, and β-catenin/Wnt. The Hippo pathway is as follows:

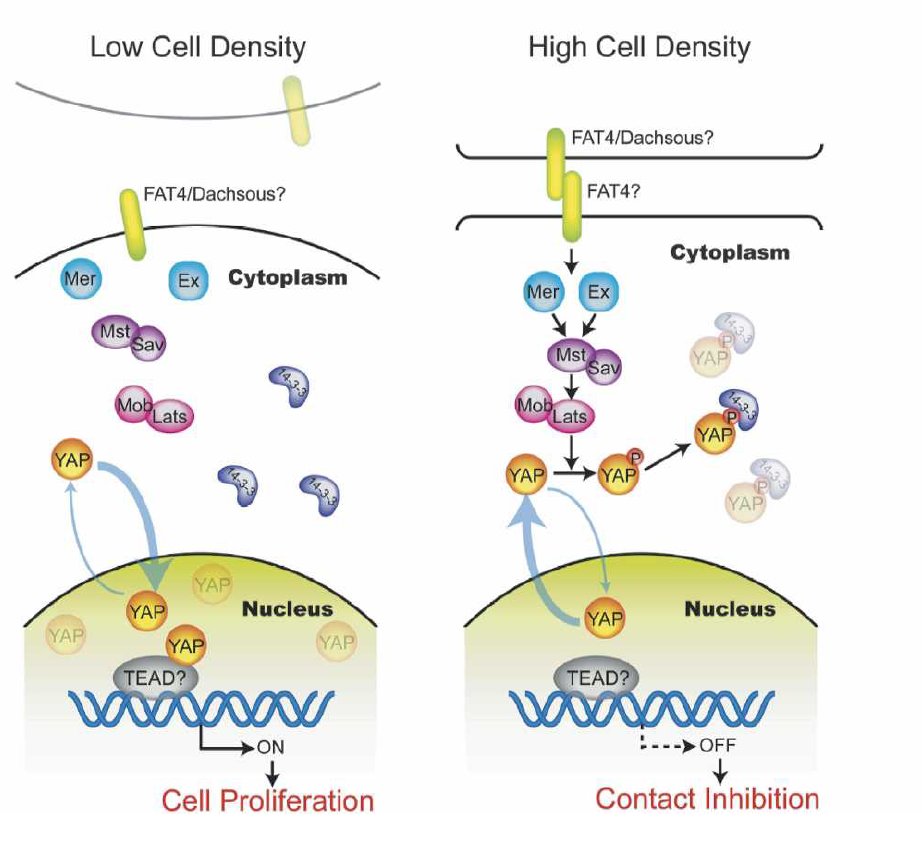
Dr. Guan notes that the Hippo pathway also controls cell contact inhibition. Based on the translocation of YAP between the nucleus and the cytoplasm in response to cell density status, Zhao et al.1 proposed a model that upon cell–cell contact, certain cell surface receptors (ie. fat) are activated via interaction with other surface proteins. As follows is a proposed model for YAP regulation by cell contact via the Hippo pathway:
NF2 is a tumor suppressor that acts upstream of the Hippo pathway, which is present in several tumors, including schwannomas, meningiomas, ependymomas, mesothelioma, lung adenocarcinoma, breast invasive ductal carcinoma, bladder urothelial carcinoma, and papillary renal cell carcinoma.4 TEAD, which is downstream in the pathway, may be a potential drug target for the aforementioned malignancies. The paradigm for regulation of the Hippo pathway includes phosphorylation-dependent nucleocytoplasmic shuttling of YAP/TAZ through a complex network of upstream components. Based on work from Lin et al.,5 showed that environmental stress promotes TEAD cytoplasmic translocation via p38 MAPK in a Hippo-independent manner. Importantly, stress-induced TEAD inhibition predominates YAP-activating signals and selectively suppresses YAP-driven cancer cell growth:
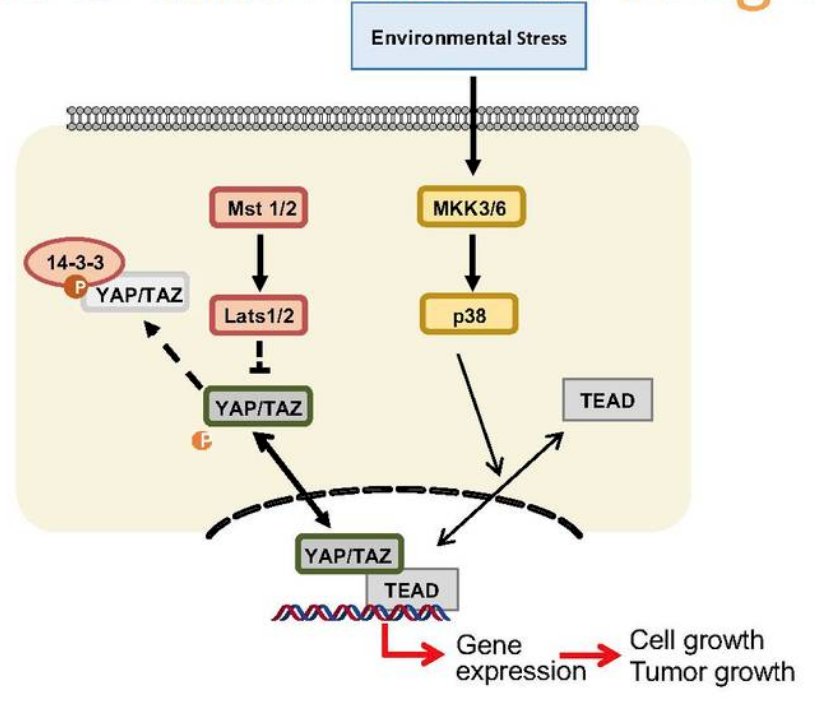
As such, this data suggests a mechanism governing TEAD nucleocytoplasmic shuttling and shows that TEAD localization is a critical determinant of Hippo signaling output, a potential target for targeted drug therapy.
Dr. Guan concluded by highlighting a study from his group6 that described the discovery of compounds that selectively inhibit YAP/TAZ-TEAD promoted gene transcription, block TEAD auto-palmitoylation, and disrupt the interaction between YAP/TAZ and TEAD. Optimization led to potent analogs (ie. VT103) with excellent oral bioavailability and pharmacokinetics that selectively inhibit NF2-deficient mesothelioma cell proliferation in vitro and growth of subcutaneous tumor xenografts in vivo. These potent and selective TEAD inhibitors provide a way to target the Hippo-YAP pathway, which is dysregulated frequently in YAP-driven cancers and diseases.
Presented by: Kun-Liang Guan, Department of Pharmacology, University of California San Diego, La Jolla, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the International Kidney Cancer Symposium (IKCS) 2021 Annual Congress, November 5 and 6, 2021.
References:
- Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007 Nov 1;21(21):2747-2761.
- Nishioka N, Inoue KI, Adachi K, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009 Mar;16(3):398-410.
- Sanchez-Vega F, Mina M, Armenia J, et al. Oncogenic signaling pathways in The Cancer Genome Atlas. Cell 2018 Apr 5;173(2):321-337.
- Ploueffe S, Meng Z, Lin KC, et al. Characterization of Hippo Pathway Components by Gene Inactivation. Mol Cell. 2016 Dec 1;64(5):993-1008.
- Lin KC, Moroishi T, Meng Z, et al. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat Cell Biol. 2017 Jul 28;19(8):996-1002.
- Tang TT, Konradi AW, Feng Y, et al. Small molecule inhibitors of TEAD auto-palmitoylation selectively inhibit proliferation and tumor growth of NF2-deficient mesothelioma. Mol Cancer Ther 2021 Jun;20(6):986-998.


