When the InPACT trial was designed in 2011, there were several important questions that were at the forefront of penile cancer management:
- Neoadjuvant therapy – What is the role of neoadjuvant therapy? Does either chemotherapy or chemoradiation provide superior outcomes?
- Treatment of the pelvis – Among patients with prior inguinal lymphadenectomy and adverse features receiving chemoradiation, what is the role of prophylactic pelvic lymph node dissection? Particularly for patients with ≥3 positive nodes and extranodal extension and those with bilateral metastases
Inclusion criteria for the InPACT trial included (i) men ≥18 years of age, (ii) biopsy-proven squamous cell carcinoma of the penis, (iii) clinical T any, N1-N3, (iv) measurable disease by RECIST criteria (≥1.5 cm short-axis diameter), (v) ECOG performance status 0-2, (vi) patients able/willing to be randomized given preoperative parameters, (vii) laboratory evaluation consistent with potential randomization to various treatment arms (ie. adequate hematologic, renal, and liver function), (viii) willing and able to comply with the follow-up schedule, and (ix) written informed consent. Key exclusion criteria included (i) pure verrucous carcinoma, (ii) squamous cell carcinoma of the urethra, (iii) non-squamous penile malignancy, (iv) stage M1 penile carcinoma, (v) prior chemotherapy or chemoradiotherapy outside of the InPACT trial, (vi) concurrent malignancy (other than SCC skin or basal cell carcinoma) that required any treatment in the last three years (low-risk prostate cancer on active surveillance was allowed), (vii) sexually active patients who are unwilling to use effective contraception. The InPACT trial design is as follows:
The primary endpoint for InPACT is survival, with secondary endpoints including disease-specific survival (DSS), disease-free survival (DFS), distant metastasis-free survival (DMFS), local recurrence-free survival (LRFS), pN0 rate, toxicity, surgical complications, and feasibility of delivering treatment on schedule. The target accrual for InPACT is 400 patients across all international sites, including 200 in North and South America. These sites are through the Eastern Co-operative of Oncology Group (ECOG), Southwest Oncology Group (SWOG), or Alliance National Clinical Trials Network (NCTN), National Cancer Institute of Canada (NCIC), and sites in Mexico and Columbia. The expected accrual is 40 patients per year for 5 years or 3-4 patients per month over 60 months; this also translates to 2 patients per site per year. As of December 2020, global accrual includes 52 participants: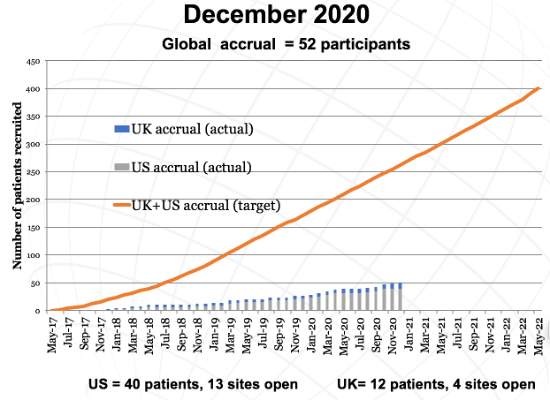
Dr. Pettaway notes that identifying appropriate sites has not been a problem, however, challenges have included:
- Credentialing urologists, radiologists, radiation oncologists, and pathologists
- COVID-19
- Outreach beyond the US and UK – so far there has been no European site participation
- Canada, Mexico, and Columbia have IRB approved sites, but low/no accrual
- India has had administrative challenges
InPACT trial contacts include Drs. Curtis Pettaway and Philippe Spiess in the US, and Drs. Steve Nicholson and Steve Penegar in the UK.
Presented by: Curtis Pettaway, MD, Professor, Department of Urology, Division of Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, Georgia, Twitter: @zklaassen_md during the 1st Global Society of Rare Genitourinary Tumors Virtual Summit, December 11-12, 2020


