(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Stephen Freedland discussing health-related quality of life in nonmetastatic hormone-sensitive prostate cancer patients with high-risk biochemical recurrence from the EMBARK study. Enzalutamide + leuprolide acetate and enzalutamide alone delayed metastasis-free survival (MFS) vs placebo + leuprolide acetate in high-risk BCR nonmetastatic hormone-sensitive prostate cancer in the phase 3 EMBARK trial:1
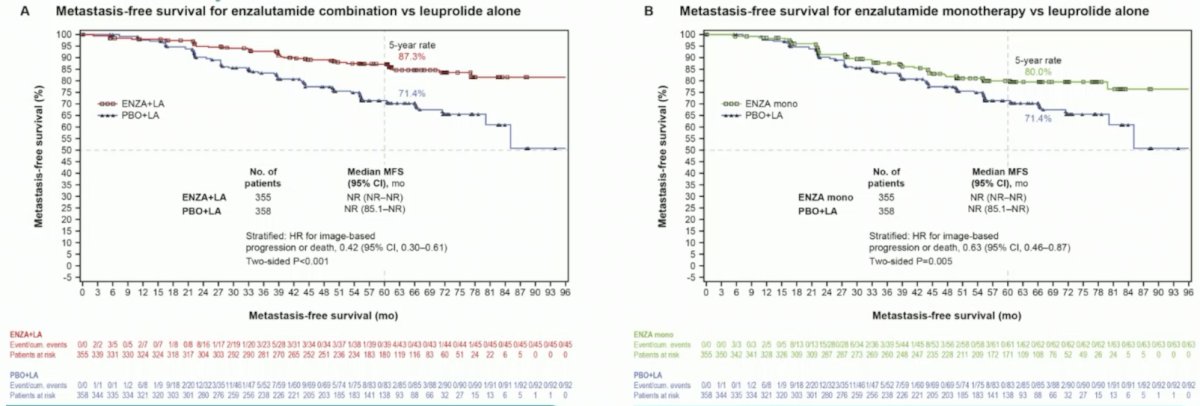
Patient-reported outcomes provide a patient perspective on disease/treatment experience not captured by clinical assessment, and Dr. Freedland presented these data at the ESMO 2023 annual congress.
In EMBARK, nonmetastatic hormone-sensitive prostate cancer patients with high-risk BCR (PSA doubling time ≤9 months, screening PSA ≥2 ng/mL above nadir post radiotherapy or ≥1 ng/mL post radical prostatectomy) were randomized (1:1:1) to enzalutamide + leuprolide acetate, enzalutamide alone, and placebo + leuprolide acetate. The trial design for EMBARK is as follows:
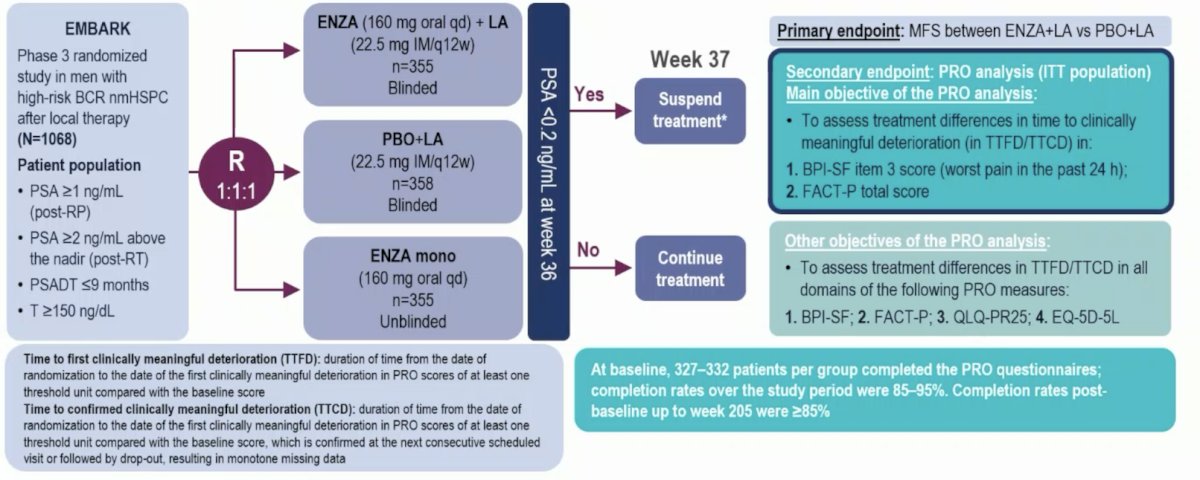
Patient-reported outcomes were assessed at baseline and every 12 weeks until the development of metastasis or death. The main objectives were to assess treatment effects in time to first deterioration and confirmed (at next visit) clinically meaningful deterioration as measured with the Brief Pain Inventory Short Form (BPI-SF) worst pain and Functional Assessment of Cancer Therapy-Prostate (FACT-P) total score using predefined thresholds. Other objectives were treatment effects in time to first deterioration and time to first clinically meaningful deterioration measured by the European Organization for Research and treatment of Cancer QoL Questionnaire-Prostate 25 (QLQ-PR25) and European QoL 5-Dimensions 5-Levels (EQ-5D-5L) visual analogue scale (VAS). Comparisons were mare for enzalutamide + leuprolide acetate vs placebo + leuprolide acetate and enzalutamide alone vs placebo + leuprolide acetate in the intent-to-treat population.
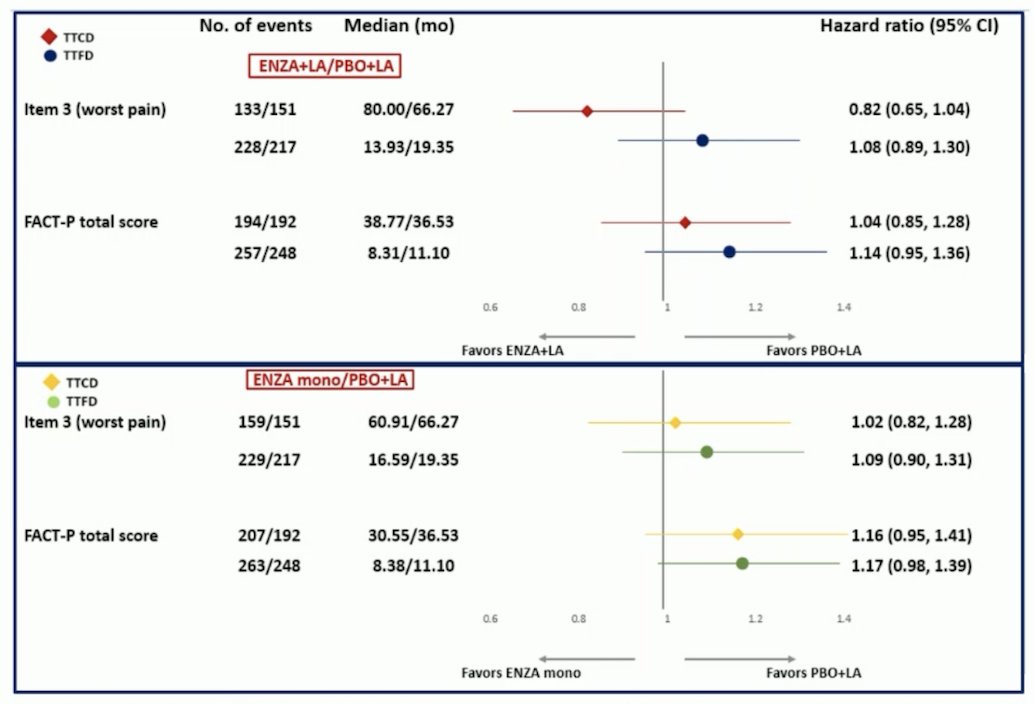
At baseline, 327–332 patients per group completed the patient reported outcome questionnaire, with 85–95% completion rates. No significant differences in time to first deterioration or time to first clinically meaningful deterioration were seen among treatment groups vs placebo + leuprolide acetate in FACT-P total score or BPI-SF worst pain:
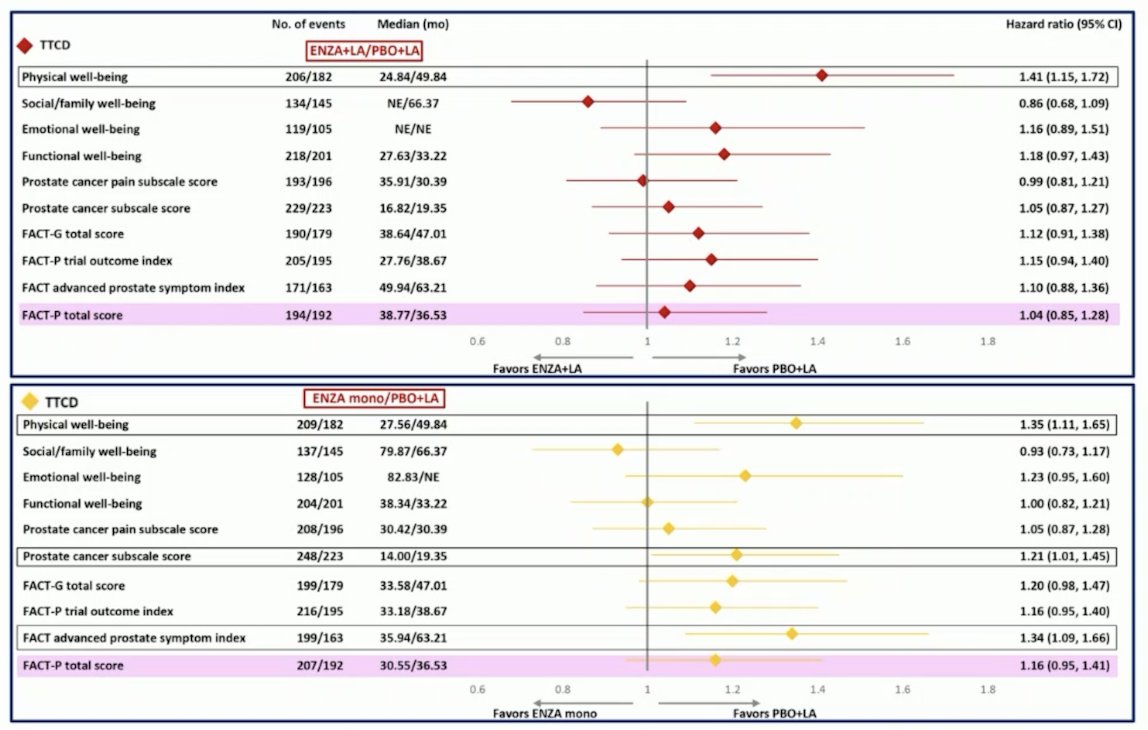
In the FACT-P subdomains, time to first clinically meaningful deterioration in the physical well-being subdomain score was significantly shorter for enzalutamide + leuprolide and for enzalutamide monotherapy versus placebo + leuprolide. Second, the median time to first clinically meaningful deterioration in the prostate cancer subscale score and advanced prostate symptom index was significantly shorter for enzalutamide monotherapy versus placebo + leuprolide. Finally, there was no significant difference observed in time to first deterioration or time to first clinically meaningful deterioration for other FACT-P subdomains:
With regards to QLQ-PR25, time to first clinically meaningful deterioration for sexual activity was significantly longer with enzalutamide alone vs placebo + leuprolide acetate. Time to first clinically meaningful deterioration for hormone treatment-related symptoms was significantly shorter with enzalutamide + leuprolide acetate vs placebo + leuprolide acetate:
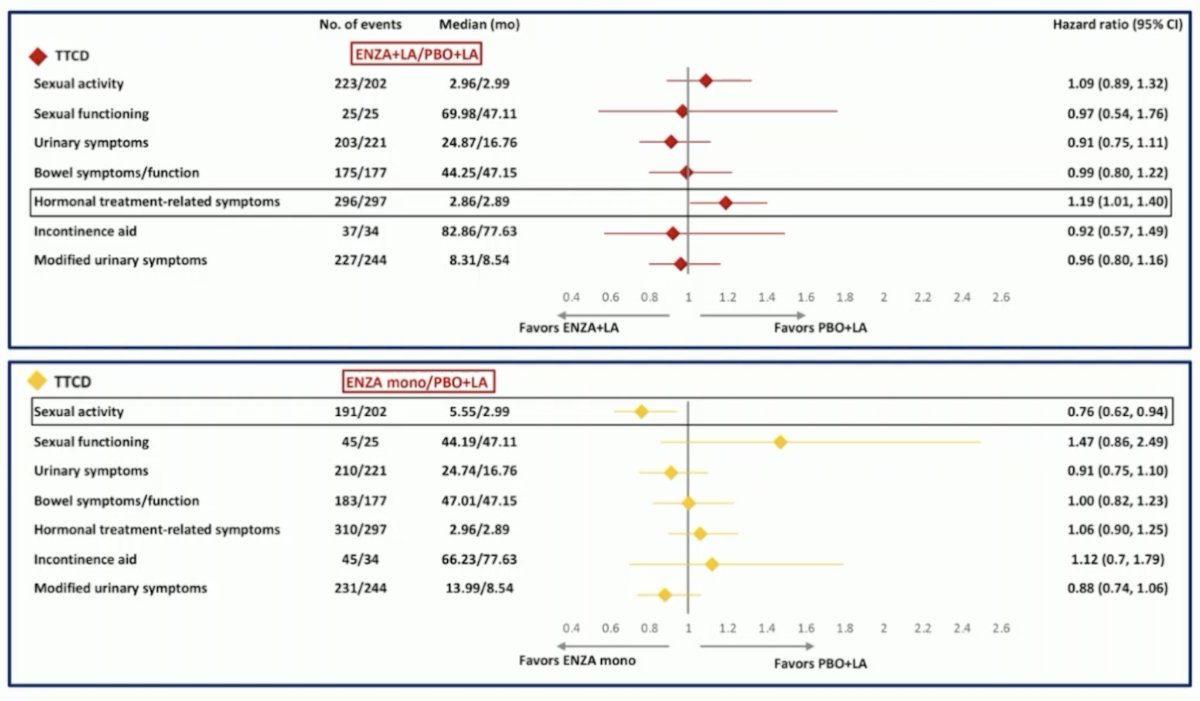
There were no significant differences observed in time to first clinically meaningful deterioration in the EQ-5D-5L VAS score in any treatment arm.
Dr. Freedland concluded his presentation by discussing health-related quality of life in nonmetastatic hormone-sensitive prostate cancer patients with high-risk biochemical recurrence from the EMBARK study with the following take-home points:
- Enzalutamide + leuprolide acetate or enzalutamide alone improved MFS without negatively impacting global health related quality of life in nonmetastatic hormone-sensitive prostate cancer patients
- There were no treatment differences in time to first deterioration or time to first clinically meaningful deterioration in the FACT-P total score and BPI-SF item 3 (worst pain in the last 24 hours)
- Hormonal treatment-related symptoms may occur earlier during treatment with enzalutamide + leuprolide than with placebo + leuprolide
- Sexual activity appears to be better preserved with enzalutamide monotherapy than with placebo + leuprolide
At the conclusion of his presentation, Dr. Freedland highlighted that the patient reported outcomes data from EMBARK were concurrently published in NEJM Evidence [2].
Presented by: Stephen J. Freedland, MD, Cedars Sinai Medical Center, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 2023 Oct 19 [Epub ahead of print].
- Freedland SJ, Gleave M, De Giorgi U, et al. Enzalutamide and quality of life in biochemically recurrent prostate cancer. NEJM Evid 2023 Oct 22 [Epub ahead of print].


