(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Henrik Grönberg discussing ProBio, a randomized, biomarker-driven trial of androgen receptor pathway inhibitors or taxanes for patients with metastatic castration-resistant prostate cancer (mCRPC). This trial is academic, outcome adaptive, and biomarker driven, randomizing patients to compare biomarker-driven treatment selection (experimental arms) against physician’s choice standard-of-care (control arm), and to compare agents against each other within the experimental treatment arms. Men with mCRPC were randomized based on genomic alterations in circulating tumor DNA in five biomarker signatures:
- AR wild type and TP53 wild type
- TP53 mutant
- DRD
- TMPRSS2:ERG fusion
- All biomarkers signatures combined
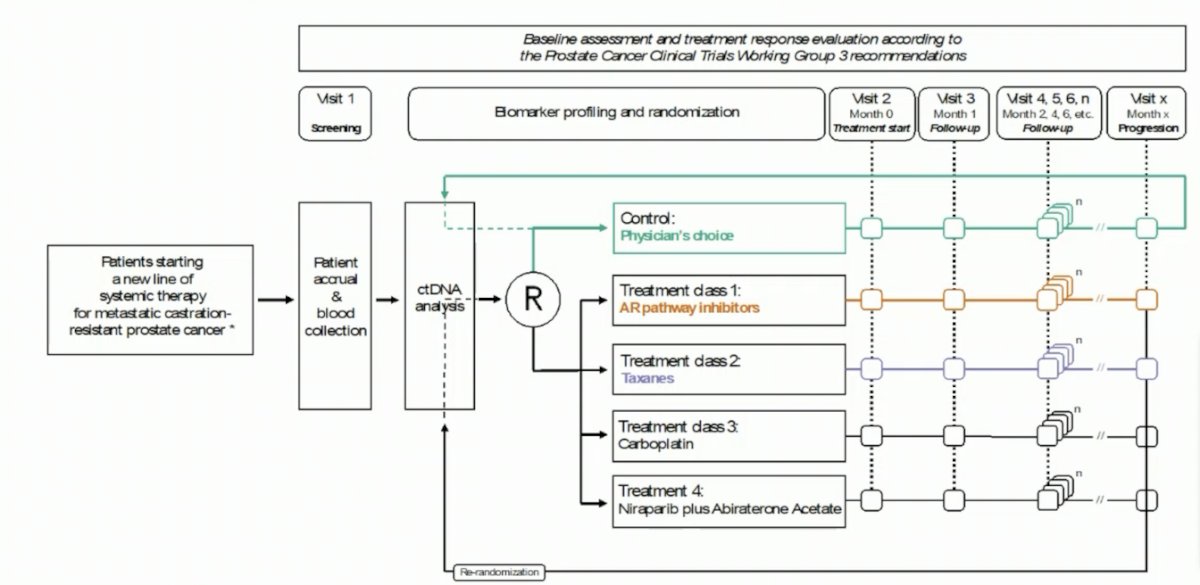
Androgen receptor pathway inhibitors (abiraterone and enzalutamide) and taxanes (docetaxel and cabazitaxel) were evaluated, using progression-free survival, by no longer clinically benefiting per PCWG3 criteria (PFS), as primary endpoint. Enrollment in the experimental group was stopped when the Bayesian probability of superiority reached prespecified thresholds.
Between February 2019 and November 2022, 343 patients with mCRPC were enrolled in ProBio and 193 men were randomized: 64 to physician’s choice, 31 to AR pathway inhibitors, and 56 to taxanes. Subsequently, 77 patients were re-randomized: 28 to physician’s choice, 20 to AR pathway inhibitors, and 19 to taxanes. The median estimated PFS was 11.1 months (90% Bayesian CI, 9.6 to 13.3) for androgen receptor pathway inhibitors compared with 7.4 months (90% CI, 6.7 to 8.4) in the physician’s choice arm and 6.9 months (90% CI 6.2 to 8.0) in the taxanes arm. The median estimated OS was 38.7 months (90% CI 31.1 to 54.1) for androgen receptor pathway inhibitors compared with 21.8 months (90% CI 19.2 to 26.3) in the physician’s choice arm, and 21.7 months (90% CI 19.0 to 26.1) in the taxanes arm:
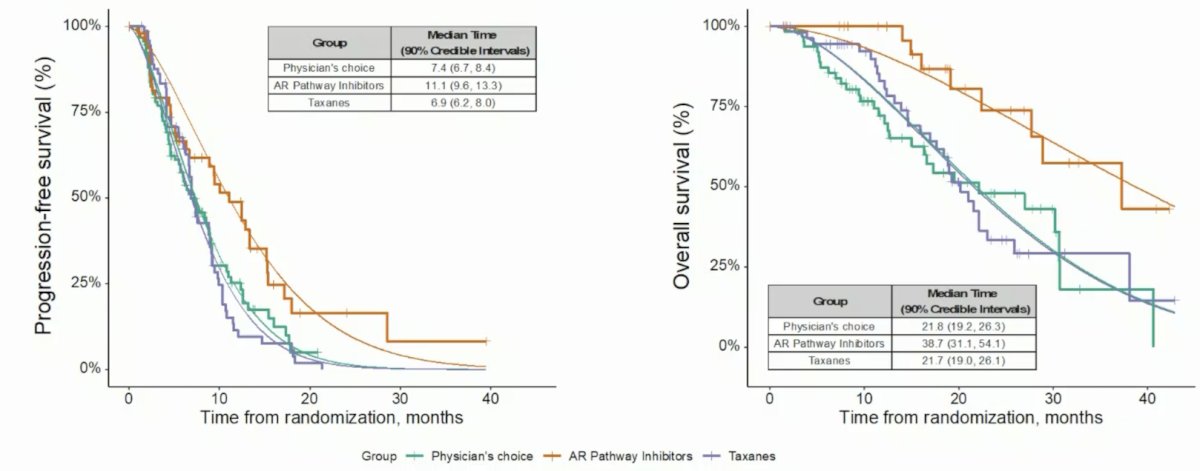
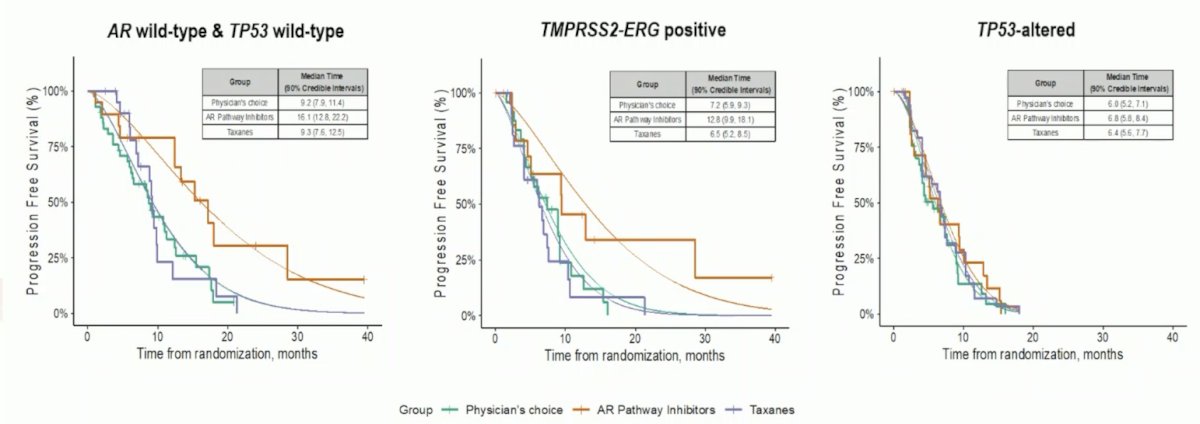
With regards to progression free survival and biomarker signatures:
- AR wild type and TP53 wild type: Median estimated PFS was 16.1 months (90% CI 12.8 to 22.2) for androgen receptor pathway inhibitors compared with 9.2 months (90% CI 7.9 to 11.4) in the physician’s choice arm and 9.3 months (90% CI 7.6 to 12.5) in the taxanes arm
- TMPRSS2-ERG positive: Median estimated PFS was 12.8 months (90% CI 9.9 to 18.1) for androgen receptor pathway inhibitors compared with 7.2 months (90% CI 5.9 to 9.3) in the physician’s choice arm and 6.5 months (90% CI 5.2 to 8.5) in the taxanes arm
- TP53-altered: Median estimated PFS was 6.8 months (90% CI 5.8 to 8.4) for androgen receptor pathway inhibitors compared with 6.0 months (90% CI 5.2 to 7.1) in the physician’s choice arm and 6.4 months (90% CI 5.6 to 7.7) in the taxanes arm
Dr. Grönberg concluded his presentation by discussing ProBio, a randomized, biomarker-driven trial of androgen receptor pathway inhibitors or taxanes for patients with mCRPC with the following take-home points:
- AR pathway inhibitors should be favored over treatment with taxanes in patients with mCRPC detectable circulating tumor DNA
- Superiority for AR pathway inhibitors was driven by patients with AR wild type and TP53 wild type, as well as TMPRSS2-ERG fusion-positive disease
- TP53-altered disease is a poor prognosis mCRPC subtype and requires new treatment strategies
- ProBio’s outcome-adaptive and biomarker-driven design shows the feasibility of using ctDNA for treatment personalization
Presented by: Henrik Grönberg, MD, Karolinska Institutet Stockholm, Sweden
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.


