(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a neoadjuvant and adjuvant therapy in genitourinary cancers session. Dr. Guillermo Antonio De Velasco Oria de Rueda delivered a state-of-the-art lecture discussing the current state of adjuvant therapy for renal cell carcinoma (RCC).
Dr. De Velasco Oria began by discussing the role of tyrosine kinase inhibitors (TKIs) in the advanced RCC space. To understand the potential role of immune checkpoint inhibitors (CPIs) for the treatment of advanced RCC, we must first shed light on the history of TKI use in advanced RCC.
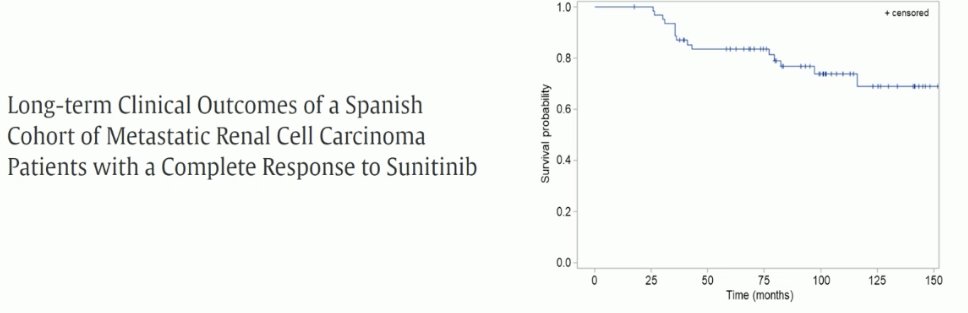
Can TKIs cure patients? Based on long-term data from a Spanish favorable cohort of metastatic RCC patients, among many other studies, the answer clearly appears to be no in the vast majority of patients.
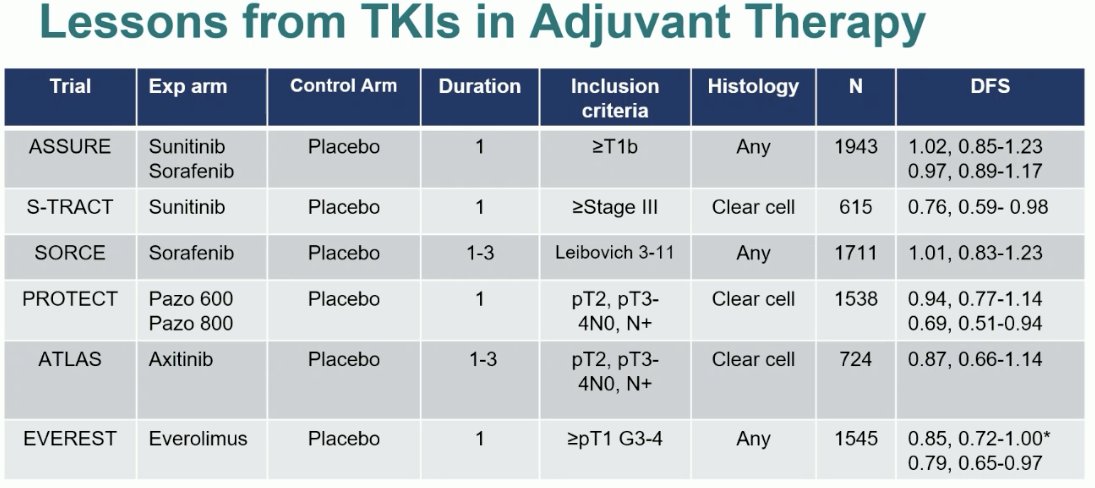
Many trials of TKIs in the adjuvant RCC setting have been performed. As summarized below, ASSURE (sorafenib/sunitinib),1 SORCE (sorafenib),2 PROTECT (pazopanib)3, ATLAS (axitinib),4 and EVEREST5 (everolimus) have all been negative trials. The only positive trial was for sunitinib in S-TRAC,6 which demonstrated a disease-free survival (DFS benefit (HR: 0.76, 95% CI: 0.59 – 0.98), but not overall survival (OS) benefit in the adjuvant setting. Nonetheless, given the paucity of options in this setting, this drug was FDA approved on the basis of this DFS benefit for clear cell RCC.
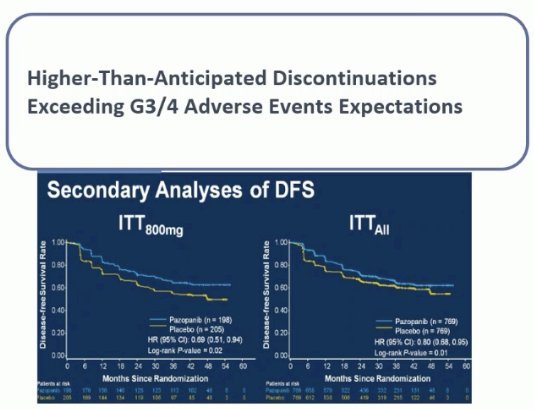
In addition to the overall lack of efficacy, there was significant toxicity with these drugs in the adjuvant setting. Additionally, it appears that dose is of utmost importance for these patients, which may come at a price of worse toxicity, with secondary DFS analysis of the PROTECT trial demonstrating that DFS was superior with the higher dose of 800 mg of pazopanib.
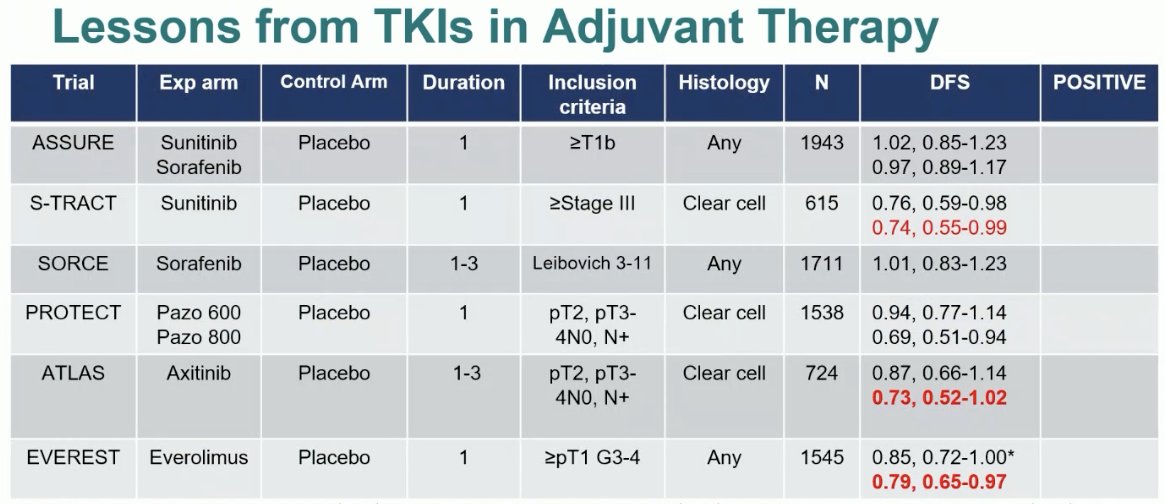
It is clear from these adjuvant TKI studies that higher risk patients derive an enhanced benefit. Patients at high risk of cancer recurrence may be more suitable for this approach. This is summarized in the table below, with higher risk patients in the S-TRAC, ATLAS, and EVERST trials deriving a higher DFS benefit compared to their lower risk counterparts. Thus, risk stratification prior to adjuvant treatment in RCC is key to better selecting eligible patients.
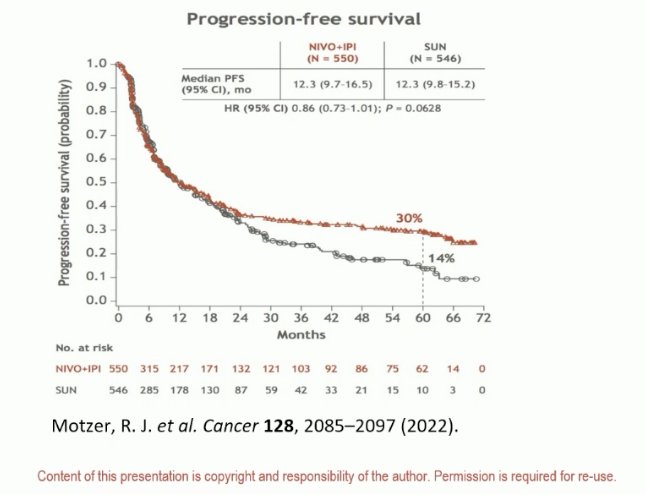
Moving on to CPIs, the same question needs to be posed for these agents: Can CPIs cure patients? If we look at long-term data from CheckMate 214, we see that approximately 30% of patients treated with ipilimumab + nivolumab experience long-term remissions.7 As such, can we translate these benefits to patients in the adjuvant setting?
What are the key findings from the phase 3 trials of CPIs in the adjuvant RCC space? These are summarized in the table below. To date, only one of these trials, KEYNOTE-564 has been positive for DFS/RFS.8
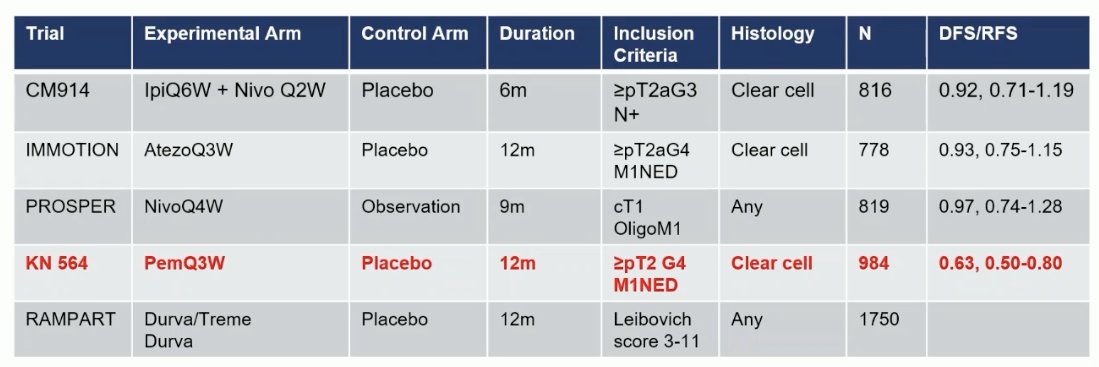
There are notable differences between these trials that may explain the observed variability in the outcomes. The CheckMate 914 trial evaluated combination ipilimumab + nivolumab.9 But in contrast to the CheckMate 214 trial in the metastatic setting, ipilimumab was given every 6 weeks in the adjuvant setting as opposed to every 3 weeks in the metastatic setting. Could this frequency discrepancy explain the negative trial results?
The duration of therapy also varied between 6 and 12 months, with 12 months the treatment duration of pembrolizumab in KEYNOTE-564. The inclusion criteria also varied as seen above, both with regard to grade/stage and histology (clear cell versus any).
KEYNOTE-564 is a double-blind, phase 3 trial, that randomized patients with clear cell RCC at high risk for recurrence after nephrectomy (within 12 weeks), with or without metastasectomy, in a 1:1 fashion to receive either adjuvant pembrolizumab 200 mg or placebo intravenously once every 3 weeks for up to 17 cycles (approximately 1 year). The primary endpoint was DFS per the investigator’s assessment. OS was a key secondary endpoint.
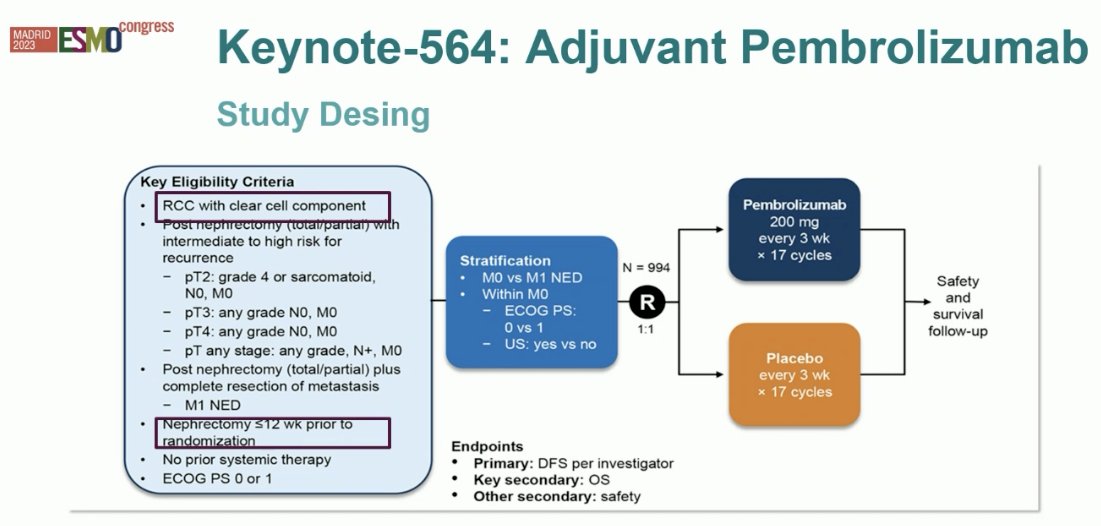
At a median follow-up of 24.1 months, pembrolizumab significantly prolonged DFS, with 24-month DFS improved from 68% to 77% (HR: 0.68, 95% CI: 0.53 – 0.87, p=0.002). The estimated percentage of patients who remained alive at 24 months was 96.6% in the pembrolizumab group and 93.5% in the placebo group (HR: 0.54; 95% CI: 0.30 - 0.96).
Based on these results the European Medical Agency (EMA) approved this drug in January 2023 for adults with RCC at an increased risk of recurrence following nephrectomy or following nephrectomy and resection of metastatic lesions.
Updated analysis later demonstrated that the PFS benefit was maintained with a HR of 0.63 (95% CI: 0.50 – 0.80), with OS benefits to date not meeting the pre-specified boundary for statistical significance of 0.000095 (HR: 0.52, p=0.0048).10
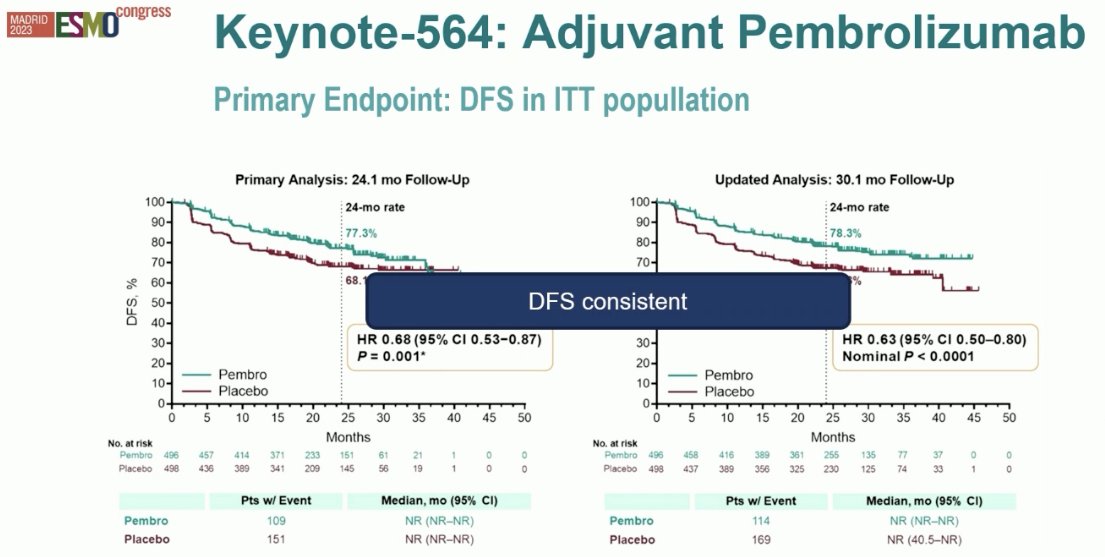
Additionally, DFS benefits were consistent across evaluable subgroups, particularly in the ’no evidence of disease’ subcohort (HR: 0.28, 95% CI: 0.12 – 0.66).
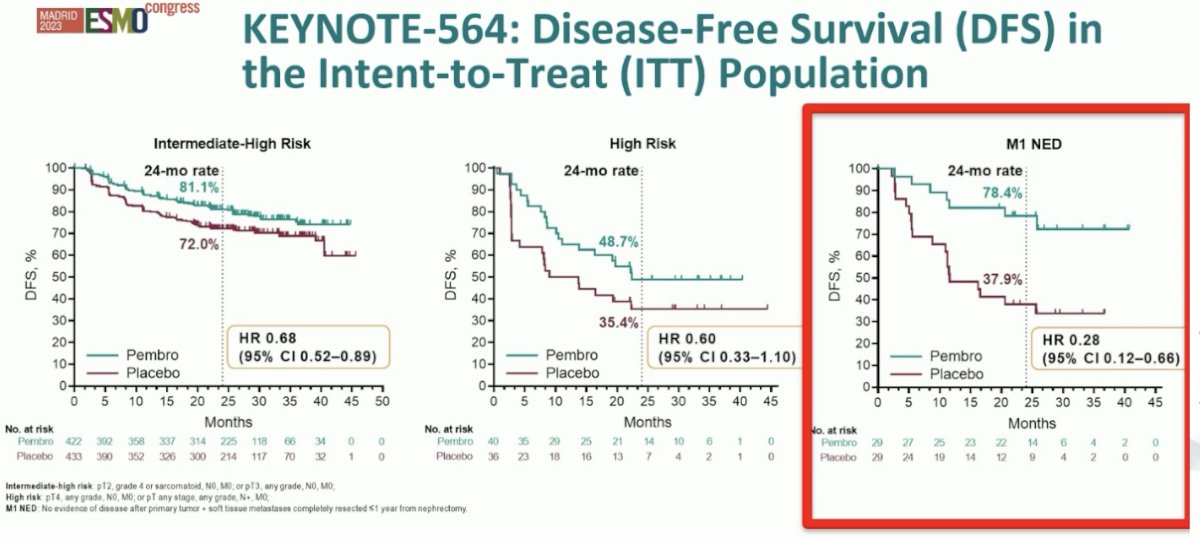
Overall, given the 8 – 9% overall improvement in DFS, it appears that the number needed to treat to prevent one DFS event in these patients is about 13. Thus, we need to weigh the benefits and risk when considering adjuvant pembrolizumab in this setting. Overall, it appears that pembrolizumab is well-tolerated in these patients, with the most common treatment-related adverse events being fatigue, pruritis, hypothyroidism, diarrhea, and rash.
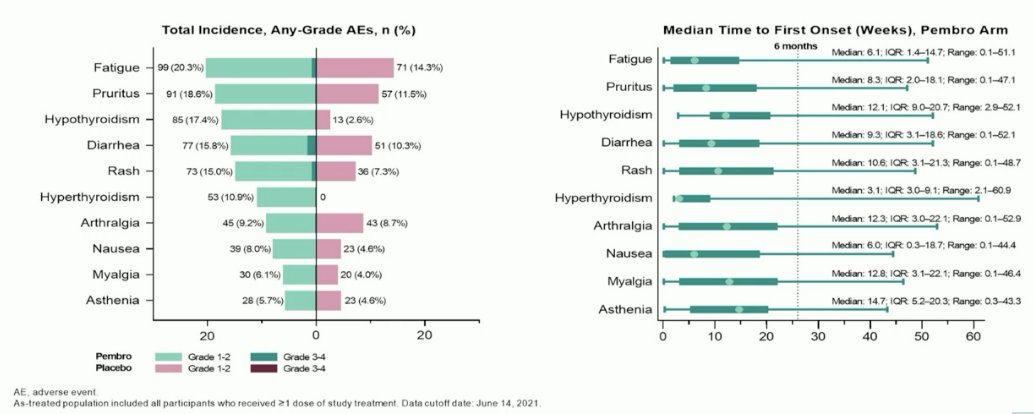
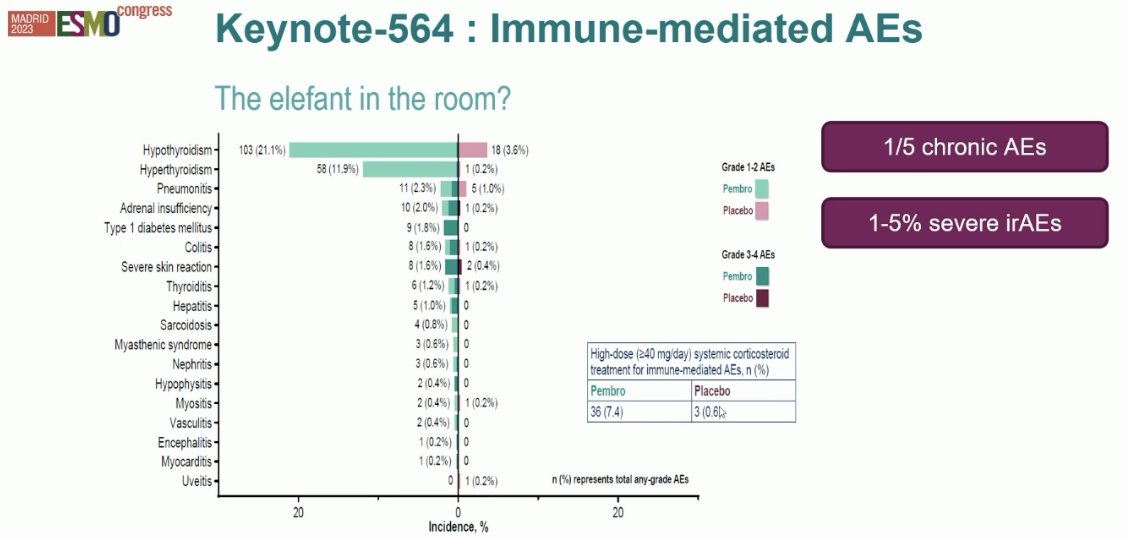
Despite this, about 20% of patients develop chronic adverse events, such as hypothyroidism. Also, 1 – 5% develop severe immune-related adverse events, such as pericarditis and colitis.
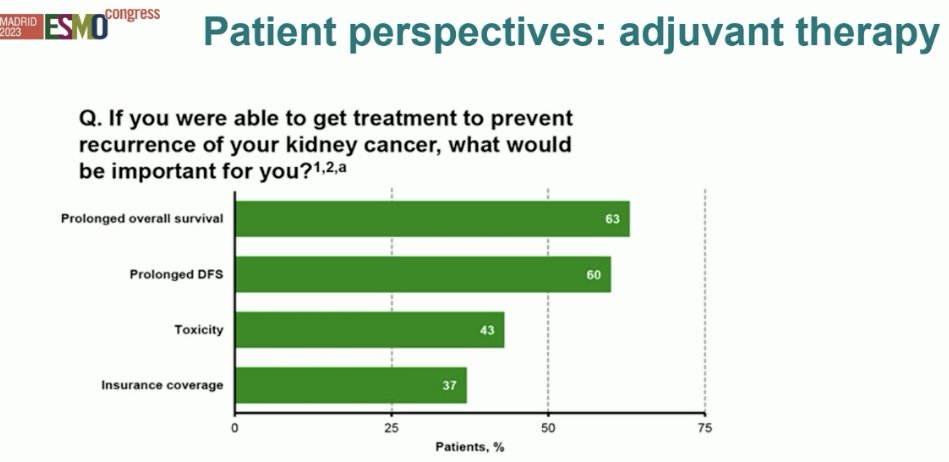
Despite EMA and FDA regulatory approval, at the current time most guidelines recommend to ‘consider’ pembrolizumab in the adjuvant RCC setting for appropriate, well-selected patients. But what do patients want? In an online survey of over 1,000 patients, 22% of whom had stage 3 RCC, most patients (67%) would receive adjuvant therapy, but almost 20% would decline. The fear of cancer and desire to eradicate any recurrences typically supersedes all other patient preferences/goals, but still, a significant proportion of these patients may decline adjuvant therapy.
Dr. De Velasco Oria concluded his presentation with the following insights and takeaways:

Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387(10032):2008-2016.
- Eisen T, Frangou E, Oza B, Ritchie AWS, et al. Adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: Results from the SORCE randomized phase III Intergroup Trial. J Clin Oncol. 2020 Dec 1;38(34):4064-4075.
- Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with locally advanced renal cell carcinoma (RCC) (PROTECT). J Clin Oncol 2017;35(35):3916-3923.
- Gross-Goupil M, Kwon TG, Eto M, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol 2018 Dec 1;29(12):2371-2378.
- Ryan CW, Tangen DM, Heath EI, et al. Adjuvant everolimus after surgery for renal cell carcinoma (EVEREST): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2023;402(10407):1043-1051.
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016;375(23):2246-2254.
- Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020 Nov;5(6):e001079.
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021 Aug 19;385(8):683-694.
- Motzer RJ, Russo P, Grunwald V, et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. Lancet. 2023;401(10379):821-832.
- Powles T, Tomczak P, Park SH, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133-1144.


