(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a non-prostate, genitourinary tumors mini oral session. Dr. Roberto Iacovelli presented the results of TIDE-A, a phase II study of avelumab plus intermittent axitinib in previously untreated patients with metastatic renal cell carcinoma (RCC).
Combinations of immune checkpoint inhibitors (CPI) with PD-1/L-1 inhibitors plus either CTLA-4 inhibitors or VEGFR-TKIs are the standard of care first line therapy for patients with mRCC across IMDC risk categories. In clinical trials of anti-PD-1/L-1 + VEGFR-TKIs, the VEGFR-TKI was reduced by 40 – 65% and discontinued by 7 – 30% of patients due to adverse events. Two studies (STAR3 and NCT011582224) have been reported and demonstrated that a TKI ‘treatment break’ is feasible in patients receiving 1st line therapy for mRCC with VEGFR-TKI alone. The aim of this study was to evaluate if patients treated with the combination of axitinib plus avelumab who achieve a tumor response can be maintained with avelumab alone in order to decrease the TKI-related toxicity and delay tumor resistance.
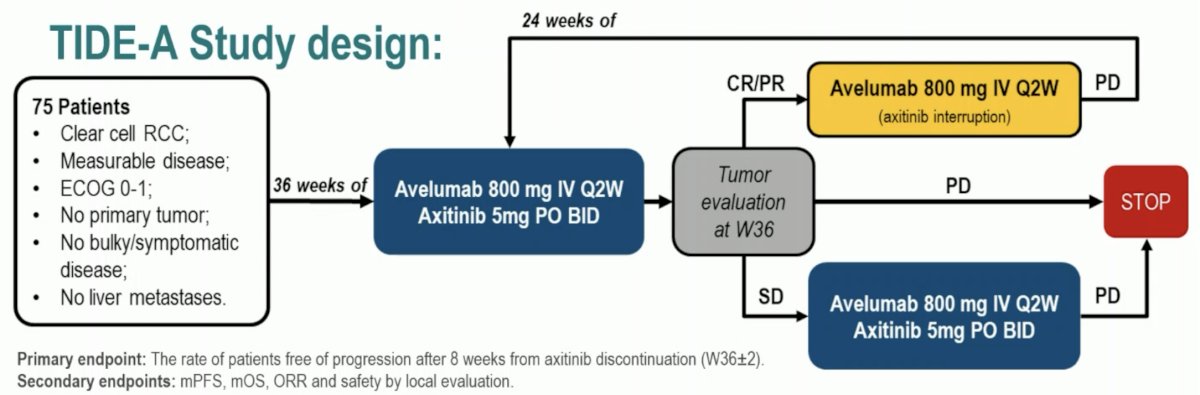
This phase II trial included 75 patients with metastatic ccRCC with no primary tumor, no bulky/symptomatic disease, and no liver metastases. These patients were planned for 36 weeks of avelumab 800 mg IV every 2 weeks + avelumab 5 mg oral twice daily. A tumor evaluation was performed at 36 weeks:
- In patients with a complete or partial response, the axitinib was discontinued and avelumab continued at the same dose every 2 weeks until evidence of progressive disease, in which case dual therapy was re-initiated
- Patients with stable disease would continue the combination until evidence of progressive disease
- Those with progressive disease would naturally discontinue this therapy
The primary endpoint was the rate of patients free of progression after 8 weeks from axitinib discontinuation. Secondary endpoints included PFS, OS, ORR, and safety by local evaluation.
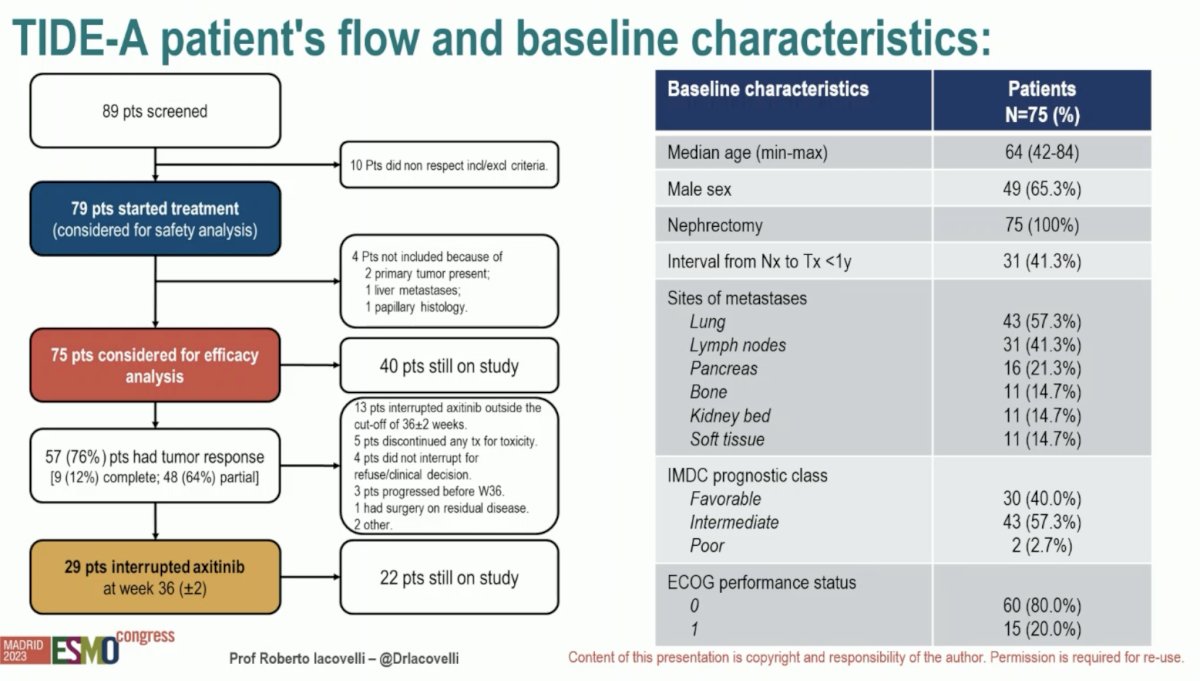
Among the 75 patients who were considered for the efficacy analysis, 57 (76%) had a tumor response (complete or partial), of whom 29 interrupted axitinib at 36 weeks. The median patient age was 64 years. All patients had undergone a nephrectomy consistent with the trial eligibility criteria. 40% of patients were IMDC favorable risk, with only 2.7% IMDC poor risk.
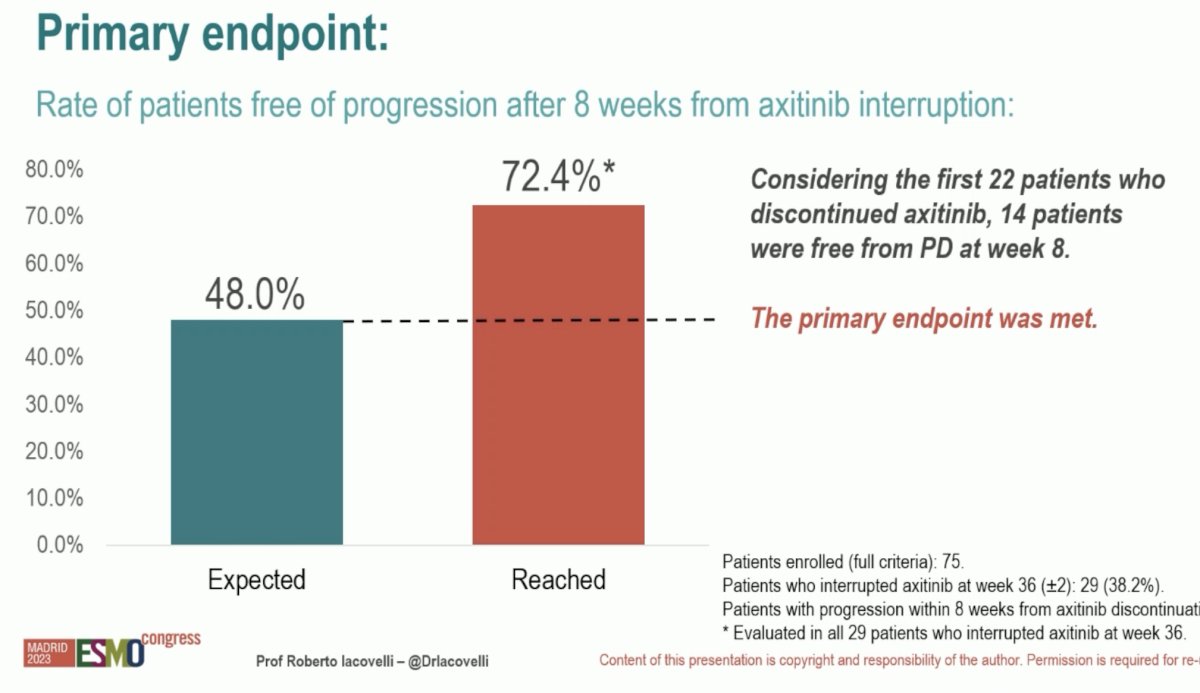
72.4% of patients were free of progression after 8 weeks of axitinib interruption.
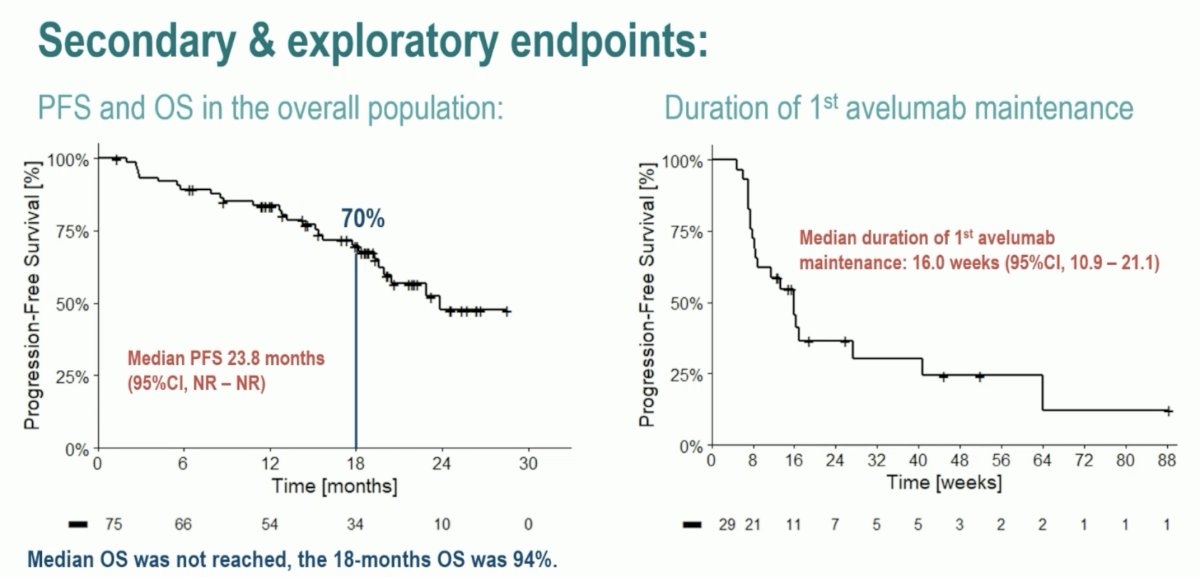
The median PFS was 24 months and median OS was not reached, with the 18-months OS at 94%. The median duration of 1st avelumab maintenance was 16 weeks.
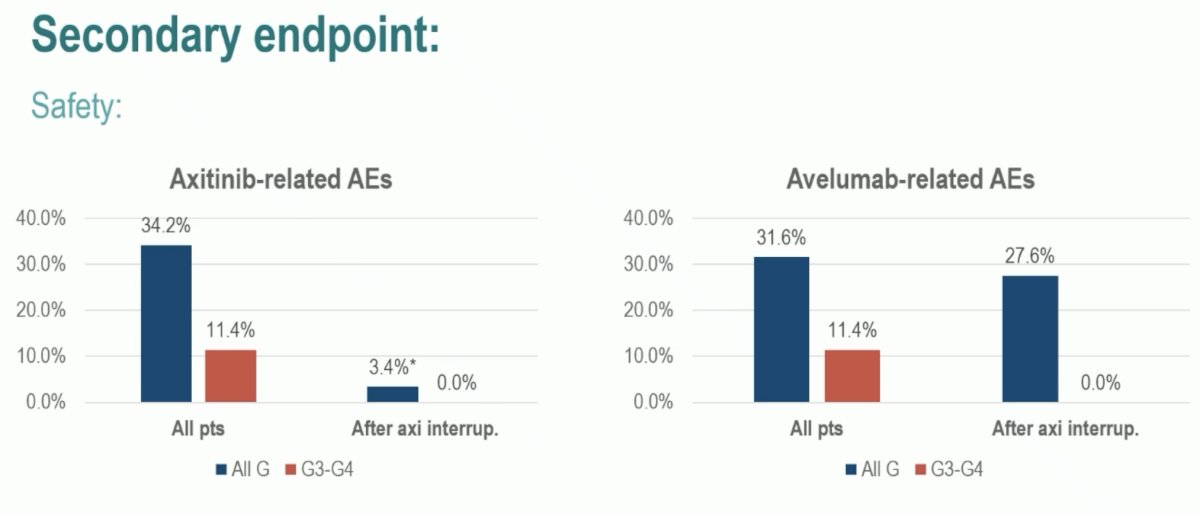
From a safety standpoint, axitinib-related any grade AEs occurred in 34% of all patients, with grade 3+ occurring in 11.4%. Following axitinib interruption, none of the patients experienced further Grade 3-4 axitinib-related AEs. Avelumab-related AEs occurred in 31.6% of patients. Notably, Grade 3 – 4 AEs occurred in 11.4% of patients receiving concurrent axitinib, and none of the patients did so following axitinib interruption.
Dr. Iacovelli concluded the presentation of the TIDE-A study as follows:
- TIDE-A is the first study showing the feasibility of the VEGFR-TKI interruption with the immunotherapy maintenance in patients with mRCC achieving maintained tumor responses following combination axitinib + avelumab therapy.
- The VEGFR-TKI interruption decreased the treatment-related toxicity while the maintenance with the immunotherapy delayed the tumor progression.
- TIDE-A confirmed the activity and safety of the combination of avelumab and axitinib in mRCC patients.
- The selection of patients (only 2.7% poor prognosis, all with nephrectomy, no liver metastases), might have impacted survival rates.
- These results warrant future investigations in a randomized trial.
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1103-1115.
- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021 Apr 8;384(14):1289-1300.
- Brown JE, Royle K, Gregory W, et al. Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): an open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2023;24(3):213-227.
- Ornstein MC, Wood LS, Elson P, et al. A Phase II Study of Intermittent Sunitinib in Previously Untreated Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2017;35(16):1764-1769.


