(UroToday.com) The 2023 ESMO annual meeting included a session on urothelial carcinoma, featuring a presentation by Dr. Russell Pachynski discussing a randomized phase II study of atezolizumab + recombinant human IL-7 (CYT107) versus atezolizumab alone in patients with locally advanced or metastatic urothelial carcinoma.
Atezolizumab is an anti-PD-L1 antibody that was approved for patients with metastatic urothelial carcinoma following platinum-containing regimens.1 IL-7 (CYT107) is a homeostatic cytokine that supports the proliferation and persistence of T cells. Previous clinical studies in cancer patients have shown that CYT107 can significantly expand lymphocyte populations. Dr. Pachynski and colleagues hypothesized that addition of CYT107 would improve responses to PD-L1 inhibition by atezolizumab. As such, they performed a randomized trial in metastatic urothelial carcinoma comparing the combination of CYT107 and atezolizumab to atezolizumab alone.
Patients with metastatic urothelial carcinoma who had progressive disease after platinum-containing chemotherapy were screened. Key eligibility criteria included ECOG PS 0-2, measurable disease (by RECIST 1.1), and no prior checkpoint inhibitor or cytokine immunotherapy. A safety run-in using the combination of CYT107 and atezolizumab was performed, followed by randomization 1:1 to atezolizumab 1200 mg IV every 3 weeks +/- CYT107 10 ug/kg IM weekly x 4 cycles: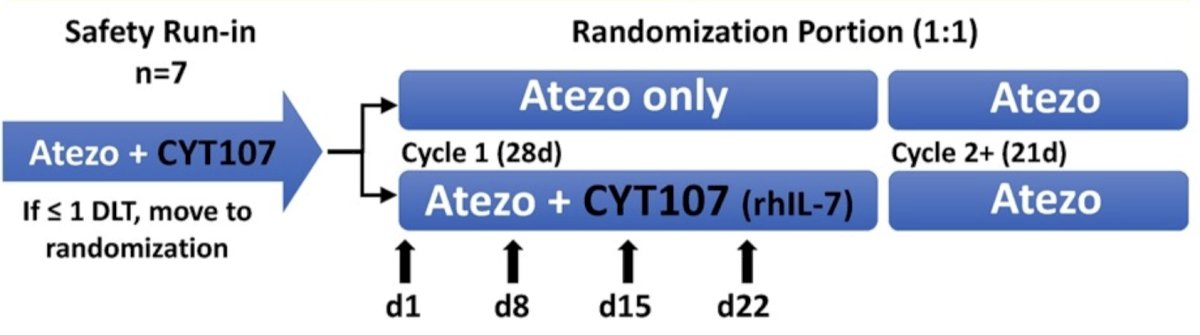
The primary endpoint was objective response rate (ORR), with secondary endpoints including clinical benefit rate, PFS, DOR, OS, and safety.
A total of 47 patients were enrolled and patient demographics between arms were well-balanced: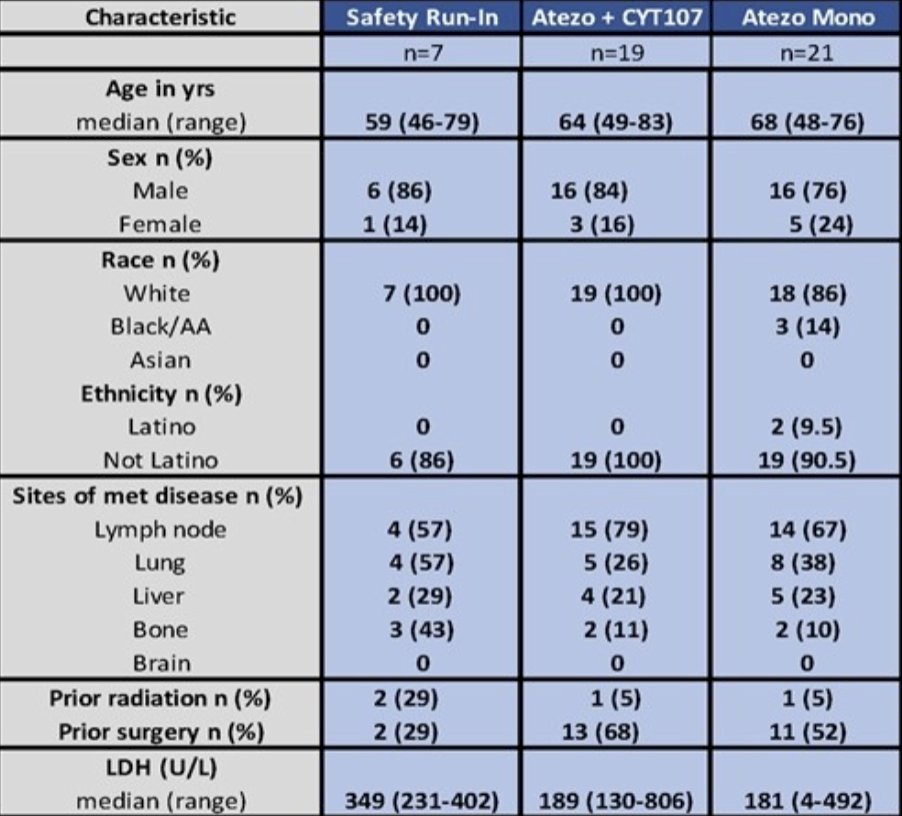
ORR was 26.3% for the combination versus 23.8% for atezolizumab alone (p=0.43):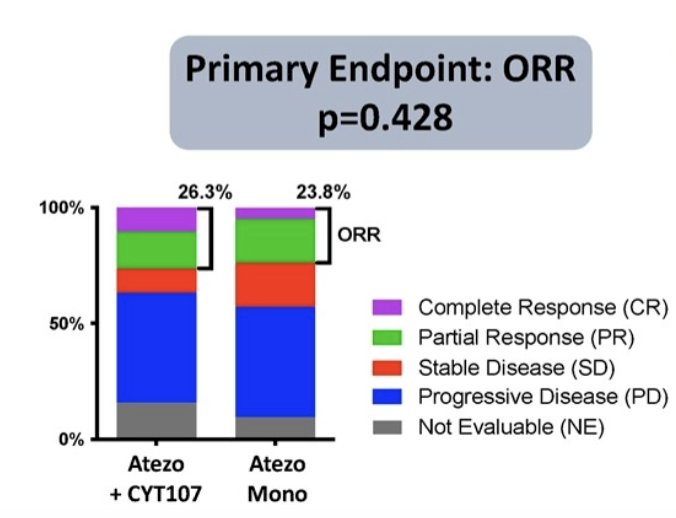
There were no significant differences in clinical benefit rate, PFS, DOR, or OS, and no dose limiting toxicities were seen in the safety run-in cohort (n = 7). Overall, 76.9% (20/26) of patients on the CYT107 arm received all four doses. The combination of CYT107 and atezolizumab was well tolerated compared to the atezolizumab arm, with overall grade 3-4 adverse events occurring in 46.2% (12/26) and 63.2% (12/19), respectively. Immune-mediated adverse events of any grade occurred in 50% (13/26) versus 68.4% (13/19), respectively. Finally, 6.3% versus 17.6% of patients on the combination vs monotherapy arm discontinued therapy due to toxicity.
Dr. Pachynski concluded his presentation discussing a randomized phase II study of atezolizumab + recombinant human IL-7 (CYT107) versus atezolizumab alone in patients with locally advanced or metastatic urothelial carcinoma with the following concluding statements:
- This is the first randomized trial of atezolizumab +/- IL-7 (CYT107) in metastatic urothelial carcinoma patients previously treated with platinum chemotherapy
- During the trial, the FDA approval for atezolizumab in metastatic urothelial carcinoma was withdrawn, which limited enrollment
- However, these results show that the addition of CYT107 to atezolizumab is safe/tolerable, although there was no improvement in clinical outcomes compared to atezolizumab monotherapy
Presented by: Russell Pachynski, MD, Siteman Cancer Center, St. Louis, MO
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
Reference:
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016;387(10031):1909-1920.


